Fig 2.
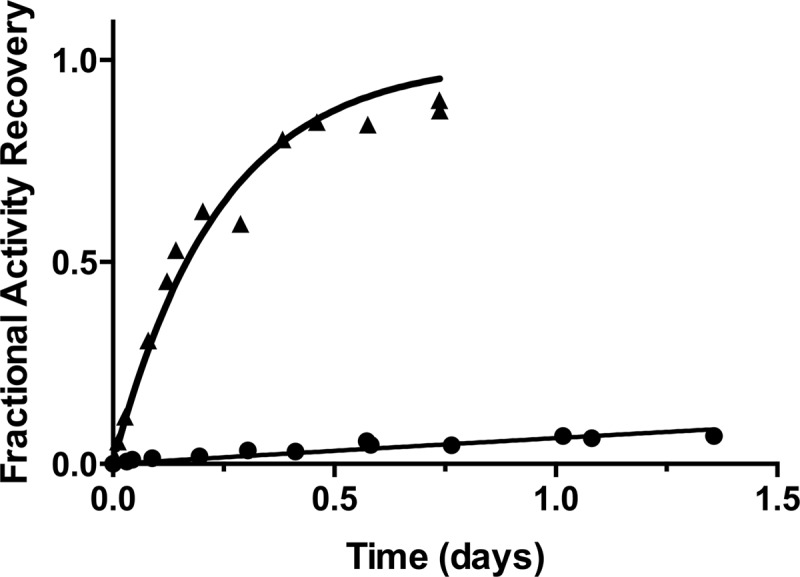
Enzymatic activity-based measurement of the slow dissociation of the BLIP-II–KPC-2 complex. In this experiment, the BLIP-II–KPC-2 complex is formed and then diluted in 200-fold excess of inactive β-lactamase competitor at time zero. The fractional activity recovery of free KPC-2 is determined by monitoring the activity over time compared to the uninhibited reaction (without BLIP-II). This recovered activity is then fit to the first-order kinetic equation (equation 2) to extrapolate the dissociation rate constant (koff). Symbols: black circles, wild-type BLIP-II; black triangles, BLIP-II Y191A mutant.
