Abstract
To provide more extensive evidence of long-term effects of vaccination on immunity against Streptococcus pneumoniae, a follow-up study of the Finnish Otitis Media (FinOM) Vaccine Trial was conducted. One of the objectives was to assess the persistence and avidity of pneumococcal antibodies 4 years after pneumococcal vaccination given in infancy. Children with complete follow-up in the FinOM trial up to 24 months of age were invited to a single visit in their fifth year of life. A blood sample was taken from all children for determination of anticapsular antibody concentrations to vaccine serotypes and avidity of antibodies to three serotypes. Children had been vaccinated at 2, 4, 6, and 12 months of age with 7-valent pneumococcal capsular polysaccharide, CRM197 conjugate vaccine (PCV7), or a control vaccine. Serum IgG antibody concentrations to vaccine serotypes remained significantly higher in children who had received PCV7 than in control children for 4 years after the fourth PCV7 dose. Concentrations of antibodies to frequently carried serotypes (6B and 19F) declined less than those of antibodies to a rarely carried serotype (4), suggesting that natural boosting contributed to antibody persistence. Furthermore, antibody avidity was significantly higher in PCV7 than control vaccine recipients. Four doses of PCV7 given in infancy elicit long-lasting antibody responses with high avidity. (This study has been registered at ClinicalTrials.gov under registration no. NCT00378417.)
INTRODUCTION
Streptococcus pneumoniae is a major causative agent of invasive disease, pneumonia, meningitis, and acute otitis media in children and adults worldwide. To date, a 7-valent pneumococcal vaccine based on conjugation of capsular polysaccharides (PS) of S. pneumoniae to a nontoxic cross-reacting variant of diphtheria toxin CRM197 (PCV7) has been proven efficacious against invasive disease (1–4), pneumonia (2, 3, 5), and acute otitis media (1, 6, 7) due to vaccine serotypes, when given in infancy, and has been licensed (Prevnar/Prevenar; Wyeth Lederle Vaccines). Following introduction of PCV7, two additional pneumococcal conjugate vaccines (PCV) were licensed on the basis of immunological noninferiority to PCV7, the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (Synflorix; GlaxoSmithKline Biologicals) (8) and 13-valent CRM197 conjugate vaccine (Prevnar 13/Prevenar 13; Wyeth Lederle Vaccines) (9). The current data on long-term effectiveness of vaccination on pneumococcal carriage and subsequent disease is, however, limited; thus, the duration of protective immunity still remains an important area of research. Despite a substantial decrease in the incidence of pneumococcal invasive and mucosal disease after 2 years of age, pneumococcus remains a significant pathogen at older ages (10, 11).
An IgG antibody concentration of 0.35 μg/ml has been used as a reference point of comparison in infant vaccine trials to support licensure of new PCVs. The IgG antibody concentration was determined from pooled data from three efficacy studies with invasive disease endpoints and measured by a World Health Organization (WHO) enzyme-linked immunosorbent assay (ELISA) (with no serotype 22F preadsorption). Its predictive value for disease episodes among individuals or for protection several years after PCV administration is unknown. Furthermore, limited data exist on persistence of pneumococcal antibodies and of the role of pneumococcal antibody avidity in long-term persistence of protective immunity. Antibody avidity, the measure of the relative strength with which antibody binds to antigen, has been shown in a murine model to inversely correlate with the amount of antibody required for prevention of lethal bacteremia and to directly correlate with improved opsonophagocytosis (12). Avidity has also been correlated with bactericidal activity (13) and with successful priming of immunological memory in humans (14, 15). Low antibody avidity has been associated with vaccine failure after Haemophilus influenzae type b (Hib) (16) conjugate vaccination. However, subjects with meningococcal serogroup C vaccine failure were not protected against disease despite having higher IgG avidity in acute-phase serum samples than unvaccinated subjects with meningococcal serogroup C disease (17).
In order to provide more extensive evidence of long-term efficacy of vaccination on pneumococcal carriage, otitis media morbidity, need for surgery because of otitis media, and immunity against pneumococcus up to 5 years of age, we conducted a follow-up study of the Finnish Otitis Media (FinOM) Vaccine Trial (6). Administration of PCV7 in infancy was associated with lower carriage rate of vaccine serotype pneumococci (8.5 versus 13.6%) in the fifth year of life (18) and with a significant reduction in tympanostomy tube placements during the period from 24 months to the fifth year of life (19).
The objective of this study was to determine serum IgG antibody concentrations to vaccine serotypes and antibody avidity to three serotypes in the fifth year of life after four doses of PCV7 or control vaccine given in infancy. The serum antibody determinations of the FinOM study (6, 20) initially were performed at the time when serotype 22F polysaccharide preadsorption (21) was not a standard practice in the enzyme immunoassay (EIA). Preadsorption with 22F is currently recommended by the WHO to improve assay specificity (22). In addition to determining antibody concentrations without 22F preadsorption, IgG antibody concentrations were measured in a subset of samples with 22F preadsorption.
MATERIALS AND METHODS
Subjects and methods.
Study vaccines. The PCV7 vaccine (Wyeth Lederle Vaccines) consisted of 2 μg of capsular polysaccharides (PS) 4, 9V, 14, 18C 19F, and 23F and 4 μg of PS 6B, each individually conjugated to the nontoxic variant diphtheria toxin (CRM197). The hepatitis B vaccine (Recombivax HB; Merck & Co., Inc.), used as a control vaccine, contained 5 μg of recombinant hepatitis B surface protein.
Vaccinees, vaccinations, and sampling.
Written informed consent was obtained from the parents/guardians of the study children prior to enrollment. The study protocol was approved by the Ethics Committee of the Pirkanmaa hospital district, Tampere, Finland. Originally, 1,662 infants were enrolled in the FinOM trial and randomized to receive either PCV7 (n = 831) or control vaccine (n = 831) at 2, 4, 6, and 12 months of age (6) (Fig. 1). A blood sample was taken at 7 or 13 months from each infant. In addition, blood samples were taken at the ages of 2, 4, 6, 7, 12, 13, and 24 months from a cohort of 115 children (57 in PCV7 and 58 in control groups). All children who had completed the trial follow-up and were still living in the study area were invited to a follow-up visit at the age of 4 to 5 years. The mean age of the participating children (n = 756) was 4.8 (range, 4.1 to 5.7) and 4.9 (range, 4.1 to 5.7) years in the PCV7 and control groups, respectively. The mean time since the last dose of study vaccine was 3.8 years (range, 3.1 to 4.8 years). At the follow-up visit, a blood sample was taken from 382 and 341 children originally in the PCV7 and control groups, respectively. A nasopharyngeal sample for detection of carriage and an unstimulated saliva sample were also taken; the data have been described elsewhere (18, 23).
Fig 1.
Process of sample selection for this study. The sets of sera selected for antibody concentration and avidity determinations, including PS 22F preadsorption, were not the same, and the sets of sera for different serotypes were not the same in these determinations.
Laboratory analyses and selection of samples.
Serum concentrations of IgG to pneumococcal capsular PSs of vaccine serotypes in samples taken at 2, 4, 6, 7, 12, 13, and 24 months and in the fifth year of life were measured by enzyme immunoassay (EIA), including preadsorption with the cell wall polysaccharide (CPS) but not with type 22F PS (24). The antibody concentrations for vaccine serotypes at 2 to 24 months of age have been described in detail elsewhere (25).
Serum concentrations of IgG to serotypes 4, 6B, and 19F were determined by EIA with CPS and 22F preadsorption (26) in a subgroup of children (Fig. 1). All children who had samples taken at 13 and 24 months and in the fifth year of life were selected to study antibody kinetics (n = 25 to 29 in the PCV7 group and n = 20 to 29 in the control group, depending on time point, serotype, and sufficient serum volume being available). Serotypes 6B and 19F were chosen because they represented relatively frequently carried serotypes (2.0 and 2.8% in the PCV7 group and 2.7 and 3.7% in the control group) as well as serotype 4, because it was rarely carried (0.3 and 0.6% in PCV7 and control groups, respectively) among 4- to 5-year-old children who were in the FinOM trial (18).
The avidity of IgG in CPS and 22F PS preadsorbed sera was measured for serotypes 4, 6B, and 19F. Avidity was measured by the thiocyanate elution assay (27), with minor modifications. Briefly, a serum dilution that yielded an optical density of 1.0 at 420 nm was calculated. Diluted sera were allowed to bind for 2 h at 37°C to an antigen-coated plate. After washing, sodium thiocyanate diluted in phosphate-buffered saline (PBS)–10% fetal bovine serum was added in duplicate at concentrations of 0 to 4 M for 15 min. After washing, the detection of remaining bound IgG was done in a manner similar to that described above for the EIA. Avidity was expressed as an avidity index (AI) corresponding to the molar concentration of sodium thiocyanate required to produce a 50% reduction in optical density. This value represents an estimation of average antibody avidity. The coefficient of variation for AI of a control serum included on each plate was ≤15%. Avidity could be determined only for sera having ≥0.5 μg/ml of serotype-specific IgG. To study the kinetics of antibody avidity, 30 children per serotype were randomly selected in the PCV7 group from children who had blood samples taken at 13 months of age and in the fifth year of life and who had serum IgG concentrations for PS 4, 6B, and 19F of ≥0.5 μg/ml at both time points (Fig. 1). In the control group, 30 children were randomly selected for serotypes 6B and 19F from children who had blood samples taken in the fifth year of life and serum IgG concentration for PS 6B and/or 19F of ≥0.5 μg/ml. For serotype 4, only 11 samples taken in the fifth year of life had serum IgG concentrations of ≥0.5 μg/ml in the control group.
Statistical methods.
Antibody concentrations are given as geometric mean concentrations (GMC) and avidity indices as geometric means (GMAI) with 95% confidence intervals (95% CI). Data are also presented as proportions of children with IgG concentrations of ≥0.35, 1.0, and 5.0 μg/ml. These levels were chosen as points of reference and do not necessarily correspond to a seroprotective level. Pearson's chi-squared test with Yates' continuity correction was applied for comparisons of proportions. Statistical comparisons were carried out using the two-sample t test on log-transformed data of concentrations and AIs. A P value of <0.05 was considered statistically significant.
RESULTS
Persistence of pneumococcal antibodies.
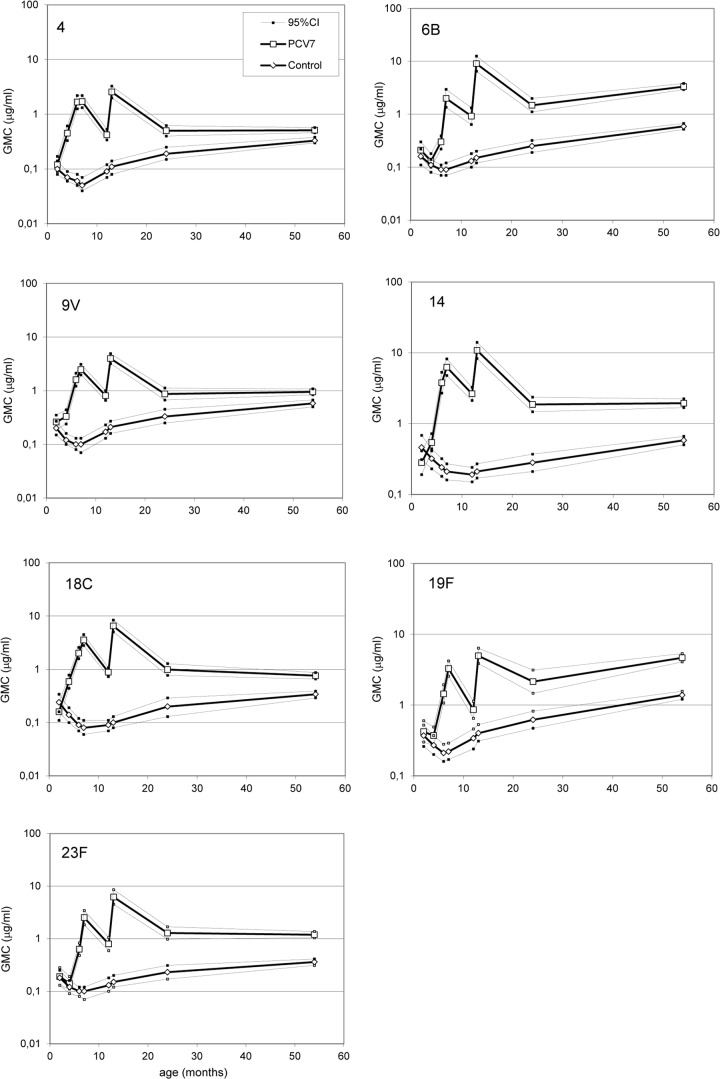
As reported previously (25), serum IgG antibodies to vaccine serotypes of PCV7 recipients show a response to primary series of three doses, the subsequent decline to the age of 1 year, a strong response to the fourth vaccine dose given at 12 months of age, and again a decline during the next 12 months (Fig. 2).
Fig 2.
Kinetics of serum pneumococcal IgG antibodies during the first 5 years of life in children immunized with PCV7 or control vaccine at the ages of 2, 4, 6, and 12 months; n (2 to 24 months) = 52 to 58; n (5 years) = 341 to 382.
The new data show that in the subsequent years, up to 4 years from the last PCV7 dose, the mean serum IgG concentrations for vaccine serotypes did not decline (Fig. 2). The GMCs of antibodies ranged between 0.51 μg/ml for serotype 4 and 4.66 μg/ml for 19F (Table 1). Furthermore, there were serotype-specific differences in the kinetics; while the GMCs of antibodies to serotypes 4, 9V, 14, 18C, and 23F remained at the 24-month level, the GMCs of antibodies to serotypes 6B and 19F were more than 2-fold higher in the fifth year of life than at 24 months of age (Fig. 2).
Table 1.
Pneumococcal IgG concentrationsa
| Serotype | GMC (95% CI) of anti-PS, μg/ml |
% of children with antibody concentrations of ≥0.35μg/ml (95% CI) |
||
|---|---|---|---|---|
| PCV7 (n = 382) | Control (n = 341) | PCV7 (n = 382) | Control (n = 341) | |
| 4 | 0.51 (0.46–0.57) | 0.33 (0.30–0.38) | 64 (59–69) | 47 (41–52) |
| 6B | 3.33 (2.92–3.81) | 0.59 (0.52–0.67) | 96 (94–98) | 70 (65–75) |
| 9V | 0.95 (0.84–1.08) | 0.58 (0.50–0.67) | 80 (76–84) | 69 (64–74) |
| 14 | 1.94 (1.68–2.24) | 0.58 (0.50–0.66) | 90 (86–92) | 64 (59–69) |
| 18C | 0.76 (0.66–0.87) | 0.34 (0.29–0.39) | 73 (68–77) | 49 (43–54) |
| 19F | 4.66 (4.07–5.34) | 1.38 (1.21–1.57) | 98 (96–99) | 86 (82–89) |
| 23F | 1.19 (1.04–1.37) | 0.36 (0.31–0.41) | 82 (78–86) | 51 (46–57) |
Results are GMC (μg/ml) and percentage of children with IgG antibody concentrations of ≥0.35μg/ml at the age of 4 to 5 years in children vaccinated with PCV7 or control vaccine at 2, 4, 6, and 12 months of age.
The data from children in the control group show the natural development of antibodies against pneumococcal PS. An increase in GMC was seen for all vaccine serotypes from 1 year of age (GMC ranging between 0.09 and 0.34 μg/ml for different serotypes) to the fifth year of life (between 0.33 and 1.38 μg/ml) (Fig. 2, Table 1). The increase was most notable for serotypes 6B, 19F, and 14, with fold increases in GMC between 2.1 and 2.4. However, the GMCs of antibodies in the fifth year of life were significantly lower in the control vaccine recipients than in the PCV7 recipients for all serotypes (P < 0.001).
The distribution of IgG concentrations in both groups in the fifth year of life can be graphically seen in the reverse cumulative distribution curves in Fig. 3. Based on the position of the curves, the seven vaccine serotypes can be divided into two groups: serotypes which show a clear difference between PCV7 and control vaccine recipients in the proportion of children with IgG concentrations above 1 and 5 μg/ml (serotypes 6B, 14, 19F, and 23F) and serotypes for which the two curves lie close to each other (serotypes 4, 9V, and 18C). The proportion of children with an IgG concentration of ≥0.35 μg/ml was significantly greater (P ≤ 0.001) for all vaccine serotypes in the PCV7 group than in the control group (Table 1 and Fig. 3).
Fig 3.
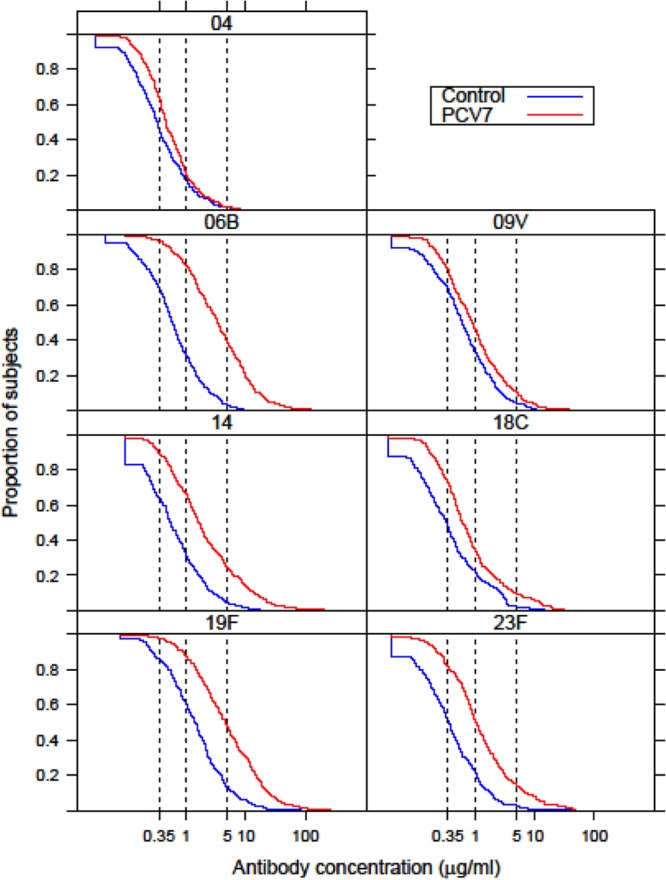
Reverse cumulative distribution curves of the serum pneumococcal IgG antibody concentrations in the fifth year of life of children who received four doses of PCV7 or control vaccine in infancy. The vertical lines denote the IgG reference concentrations of 0.35, 1.0, and 5.0 μg/ml.
Effect of PS 22F preadsorption on antibody concentrations.
The effect of PS 22F preadsorption on IgG concentrations was assessed for a subset of samples taken at 13 and 24 months and in the fifth year of life for relatively frequently carried serotypes 6B and 19F and a rarely carried serotype, serotype 4. PS 22F preadsorption resulted in greater assay specificity and lower serotype-specific responses; ratios of GMCs ranged between 1.1 and 3.1 in the PCV7 group and between 2.1 and 4.9 in the control group for all three serotypes (Fig. 4). When measured without PS 22F preadsorption, the mean IgG concentrations did not differ between the selected subset of samples and the whole study cohort (data not shown).
Fig 4.
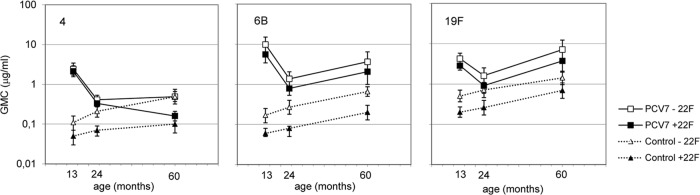
Effect of PS 22F preadsorption on IgG antibody concentration (results are GMC, in μg/ml, with 95% CI) kinetics in the PCV7 (n = 25 to 29) and control (n = 20 to 29) groups between 13 months and 5 years of age for serotypes 4, 6B, and 19F.
In the PCV7 group, PS 22F preadsorption did not change the kinetics of antibody concentrations between 13 months and 5 years of age for serotypes 6B and 19F. However, for serotype 4, preadsorption with PS 22F resulted in different kinetics, showing a decrease in GMC of IgG between the age of 24 months and 5 years of age (Fig. 4). In the control group, the GMCs of IgG after preadsorption of sera with PS 22F increased with age, but the increase was more evident for serotypes 6B and 19F than for serotype 4; fold increases in GMC between 24 months and 5 years of age were 2.5, 2.7, and 1.4, respectively. At 5 years of age, the GMC of IgG was significantly higher in the PCV7 group than in the control group for serotypes 6B and 19F (P < 0.001), whereas for serotype 4 no significant difference in GMC was found (P = 0.13) (Fig. 4).
Antibody avidity in the fifth year of life.
Due to limitations of the assay, avidity was determined only for samples with IgG concentrations of ≥0.5 μg/ml. Thus, the kinetics of IgG avidity could be studied only in the PCV7 group. The previous data of the FinOM trial for serotypes 6B and 19F show that four doses of PCV7 induce an increase in mean AI between 7 and 24 months for serotype 6B but not for 19F. At the age of 24 months the mean AI is, however, higher in the PCV7 than in the control group for both serotypes (25).
In the fifth year of life, the GMAI of IgG in the PCV7 recipients was similar to the GMAI at 13 months of age and was higher than that in the control group for serotypes 4 and 6B (Fig. 5) (P < 0.001). For serotype 19F, the GMAI of antibodies decreased between the ages of 13 months and 5 years, but in the fifth year of life it was still higher in the PCV7 than the control group (P = 0.001).
Fig 5.
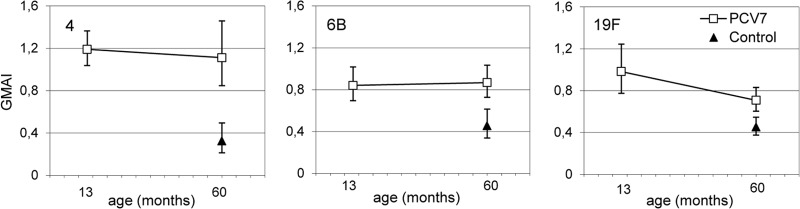
Pneumococcal IgG antibody avidity (results are GMAI, with 95% CI) kinetics between 13 months and 5 years (60 months) of age in the PCV7 (n = 30) and control (n = 11 for serotype 4, n = 30 for 6B and 19F) vaccine groups. The vaccines were administered at 2, 4, 6, and 12 months of age.
DISCUSSION
These results demonstrate that IgG antibody concentrations to vaccine serotypes remain at significant levels in children for as long as 4 years after the fourth dose of PCV7 given in infancy. Antibody concentrations to frequently carried serotypes (6B and 19F) were higher than those to rarely carried serotype 4, suggesting that natural exposure to bacteria contributed to persistence of antibodies. Furthermore, antibody avidity was significantly higher in PCV7 than in control vaccine recipients, suggesting that good functionality of antibodies persisted 4 years after the fourth PCV7 dose.
The currently available data on long-term persistence of pneumococcal antibodies after vaccination of infants and children is limited. After the introduction of PCV into national immunization programs in several parts of the world, the WHO has emphasized the importance of viewing antibody persistence in conjunction with effectiveness data (22). The current data on persistence of pneumococcal antibodies after PCV given in infancy come from only a few studies, two studies with 9-valent CRM197 conjugate vaccine conducted in South Africa (28, 29), a study with 11-valent PCV with H. influenzae protein D as a carrier conducted in the Czech Republic (30), and a study with 10-valent PCV with H. influenzae protein D as a carrier conducted in Sweden and Slovakia (31), in which the follow-up time ranged from 15 months (28) to 5 years (29, 30, 32) after primary vaccination. In addition, phase II studies with investigational 4- or 8-valent PCV formulations included follow-up until the age of 2 (33) to 3 (34) years, but these studies did not include comparison to unvaccinated controls. The results of the present study are in agreement with the existing data.
It is important to realize that the currently available data (including the data presented here) on persistence of pneumococcal antibodies after administration of PCV come from studies conducted before the introduction of PCV into national immunization programs. After the introduction of PCV into national immunization programs, the natural exposure of the population to pneumococcal serotypes included in the vaccine is expected to decrease, which is likely to affect the persistence of pneumococcal antibodies in the population. In the present study, this could be illustrated by concentrations of antibodies to the rarely carried serotypes 4 and 9V, which did not increase between 24 months and 5 years of age in the PCV7 group, in contrast to concentrations of antibodies to the more frequently carried serotypes 6B and 19F, yet they were higher than levels in the control group. This suggests that the higher antibody levels against relatively frequently carried serotypes were the result of natural exposure, including nasopharyngeal colonization by vaccine serotypes or bacteria with cross-reactive epitopes. Furthermore, during this period there was also a notable increase for the same serotypes in antibody levels of the unvaccinated children. More data will be essential for assessment of persistence of pneumococcal antibodies after introduction of PCV into national immunization programs.
The EIA determinations of this study were performed without polysaccharide 22F preadsorption to allow comparison of the follow-up antibody levels to the previously determined antibody levels in the FinOM study. Polysaccharide 22F preadsorption (21) was not a standard practice in the EIA at the time the antibody determinations of the FinOM study were initially performed. It is, however, important to properly address analytical specificity to recognize the immunogenic cell wall polysaccharide (CPS), non-CPS polysaccharide, and protein contaminants found in the purified pneumococcal polysaccharides used in pneumococcal vaccines and in the EIA. To reduce such non-serotype-specific responses, the addition of PS 22F to the serum diluent before performing EIA is currently recommended (21, 22). To evaluate the effect of 22F preadsorption on antibody concentrations and kinetics in this study, a subset of sera was reanalyzed after preadsorption with PS 22F. The greater assay specificity resulted in a change in IgG antibody concentration kinetics for serotype 4 only. This shows that the increase in antibody concentrations until the fifth year of life was due to specific antibodies for serotypes 6B and 19F in children who had received PCV7 or control vaccine in infancy. In contrast, the specific mean IgG antibody concentration for serotype 4 decreased in the PCV7 group and stayed similar in the control group at 13 months and 5 years of age, which may be due to relatively low exposure to natural contact with serotype 4 in the population. The results suggest that natural exposure to serotype-specific bacteria contributes to the persistence of antibody levels in previously vaccinated and unvaccinated subjects.
In addition, the data of this study show that IgG antibodies detected 4 years after PCV7 given in infancy have higher mean avidity than antibodies of unvaccinated controls. The higher avidity antibodies in the PCV recipients may have been produced by B cells that have, following a T cell-dependent antigen stimulus, such as PCV, undergone a selection process in the germinal centers during which they have somatically mutated their Ig V region genes to generate high-affinity memory B cells and long-lived plasma cells that have given rise to long-term serum antibodies. In contrast, in the children of the control group, the antibodies have been generated after natural encounter by pneumococcus or cross-reacting pathogen without induction of B cell maturation and affinity maturation. The data on Hib vaccine studies suggests that high-avidity antibodies have better functional activity than antibodies with low avidity (13), but the importance of avidity in determining protection from disease is unclear.
Our study has some limitations. Because antibody avidity could reliably be determined only for sera having ≥0.5 μg/ml of anti-PS IgG after preadsorption with 22F PS, the set of sera in the avidity determinations was selected, and it was not necessarily a representative sample of the whole study population. This was emphasized in the control group, in which antibody concentrations generally were lower than those in vaccinated children. It can be speculated that in the control group, the sera selected for the avidity measurements were from individuals who had had previous contact with specific or cross-reacting pathogen and consequently had serum antibodies against S. pneumoniae. In that case, the differences in immune responses between PCV7 and control groups in this study would reflect the differences between PCV-induced and natural priming of the immune system. Another limitation of the study was that for each study group, evaluation of (22F EIA) IgG antibody concentrations and antibody avidity for each of the serotypes (4, 6B, and 19F) was based on sera from different subsets of subjects (total of 12 subsets). Furthermore, the relatively small number of available sera for anti-serotype 4 antibody avidity determinations in the control group was a limiting factor of the study.
The results presented here lead us to conclude that PCV7 given in infancy elicits serum antibodies which are of high avidity and which are maintained at significant levels at least 4 years after the fourth vaccine dose.
ACKNOWLEDGMENTS
This work was supported by Aventis Pasteur, Merck & Co., Inc., and Wyeth Vaccines.
We thank Mika Lahdenkari for statistical advice, Esa Ruokokoski for experienced data management, the personnel of the study health centers, and all the children and their parents who volunteered to participate in the FinOM study.
Footnotes
Published ahead of print 8 May 2013
REFERENCES
- 1. Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G, Malinoski F, Madore D, Chang I, Kohberger R, Watson W, Austrian R, Edwards K. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 19:187–195 [DOI] [PubMed] [Google Scholar]
- 2. Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, Okoko JB, Oluwalana C, Vaughan A, Obaro SK, Leach A, McAdam KP, Biney E, Saaka M, Onwuchekwa U, Yallop F, Pierce NF, Greenwood BM, Adegbola RA. 2005. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 365:1139–1146 [DOI] [PubMed] [Google Scholar]
- 3. Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 349:1341–1348 [DOI] [PubMed] [Google Scholar]
- 4. O'Brien KL, Moulton LH, Reid R, Weatherholtz R, Oski J, Brown L, Kumar G, Parkinson A, Hu D, Hackell J, Chang I, Kohberger R, Siber G, Santosham M. 2003. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet 362:355–361 [DOI] [PubMed] [Google Scholar]
- 5. Black S, Shinefield H. 2002. Safety and efficacy of the seven-valent pneumococcal conjugate vaccine: evidence from northern California. Eur. J. Pediatr. 161(Suppl. 2):S127–S131 [DOI] [PubMed] [Google Scholar]
- 6. Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, Herva E, Takala A, Käyhty H, Karma P, Kohberger R, Siber G, Mäkelä PH. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403–409 [DOI] [PubMed] [Google Scholar]
- 7. Fireman B, Black SB, Shinefield HR, Lee J, Lewis E, Ray P. 2003. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr. Infect. Dis. J. 22:10–16 [DOI] [PubMed] [Google Scholar]
- 8. Prymula R, Schuerman L. 2009. 10-Valent pneumococcal nontypeable Haemophilus influenzae PD conjugate vaccine: Synflorix. Expert Rev. Vaccines 8:1479–1500 [DOI] [PubMed] [Google Scholar]
- 9. Bryant KA, Block SL, Baker SA, Gruber WC, Scott DA. 2010. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine. Pediatrics 125:866–875 [DOI] [PubMed] [Google Scholar]
- 10. Black S, Eskola J, Whitney C, Shinefield H. 2008. Pneumococcal conjugate vaccine and pneumococcal common protein vaccines. In Plotkin SA, Orenstein WA, Offit PA. (ed), Vaccines, 5th ed. Saunders Elsevier, Philadelphia, PA [Google Scholar]
- 11. O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902 [DOI] [PubMed] [Google Scholar]
- 12. Usinger WR, Lucas AH. 1999. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect. Immun. 67:2366–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schlesinger Y, Granoff DM. 1992. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. The Vaccine Study Group. JAMA 267:1489–1494 [PubMed] [Google Scholar]
- 14. Goldblatt D, Vaz AR, Miller E. 1998. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J. Infect. Dis. 177:1112–1115 [DOI] [PubMed] [Google Scholar]
- 15. Joseph H, Miller E, Dawson M, Andrews N, Feavers I, Borrow R. 2001. Meningococcal serogroup A avidity indices as a surrogate marker of priming for the induction of immunologic memory after vaccination with a meningococcal A/C conjugate vaccine in infants in the United Kingdom. J. Infect. Dis. 184:661–662 [DOI] [PubMed] [Google Scholar]
- 16. Lee YC, Kelly DF, Yu LM, Slack MP, Booy R, Heath PT, Siegrist CA, Moxon RE, Pollard AJ. 2008. Haemophilus influenzae type b vaccine failure in children is associated with inadequate production of high-quality antibody. Clin. Infect. Dis. 46:186–192 [DOI] [PubMed] [Google Scholar]
- 17. Auckland C, Gray S, Borrow R, Andrews N, Goldblatt D, Ramsay M, Miller E. 2006. Clinical and immunologic risk factors for meningococcal C conjugate vaccine failure in the United Kingdom. J. Infect. Dis. 194:1745–1752 [DOI] [PubMed] [Google Scholar]
- 18. Palmu A, Kaijalainen T, Verho J, Herva E, Mäkelä PH, Kilpi T. 2002. Long-term efficacy of the sevenvalent PncCRM vaccine on nasopharyngeal carriage, abstr 24. Abstr. 3rd Int. Symp. Pneumococci Pneumococcal Dis [Google Scholar]
- 19. Palmu AA, Verho J, Jokinen J, Karma P, Kilpi TM. 2004. The seven-valent pneumococcal conjugate vaccine reduces tympanostomy tube placement in children. Pediatr. Infect. Dis. J. 23:732–738 [DOI] [PubMed] [Google Scholar]
- 20. Kilpi T, Åhman H, Jokinen J, Lankinen KS, Palmu A, Savolainen H, Grönholm M, Leinonen M, Hovi T, Eskola J, Käyhty H, Bohidar N, Sadoff JC, Mäkelä PH. 2003. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin. Infect. Dis. 37:1155–1164 [DOI] [PubMed] [Google Scholar]
- 21. Concepcion NF, Frasch CE. 2001. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8:266–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organization 2009. Recommendations to assure the quality, safety and efficacy of pneumococcal conjugate vaccines. Proposed replacement of Technical Report Series no. 927, annex 2, ECBS. World Health Organization, Geneva, Switzerland [Google Scholar]
- 23. Nurkka A, Lahdenkari M, Palmu A, Käyhty H. 2004. Salivary antibodies induced by the seven-valent PncCRM conjugate vaccine in the Finnish Otitis Media Vaccine Trial. Vaccine 23:298–304 [DOI] [PubMed] [Google Scholar]
- 24. Käyhty H, Åhman H, Rönnberg PR, Tillikainen R, Eskola J. 1995. Pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine is immunogenic in infants and children. J. Infect. Dis. 172:1273–1278 [DOI] [PubMed] [Google Scholar]
- 25. Ekström N, Åhman H, Verho J, Jokinen J, Väkeväinen M, Kilpi T, Käyhty H. 2005. Kinetics and avidity of antibodies evoked by heptavalent pneumococcal conjugate vaccines PncCRM and PncOMPC in the Finnish Otitis Media Vaccine Trial. Infect. Immun. 73:369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simell B, Lahdenkari M, Reunanen A, Käyhty H, Väkeväinen M. 2008. Effects of ageing and gender on naturally acquired antibodies to pneumococcal capsular polysaccharides and virulence-associated proteins. Clin. Vaccine Immunol. 15:1391–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goldblatt D, Southern J, Ashton L, Richmond P, Burbidge P, Tasevska J, Crowley-Luke A, Andrews N, Morris R, Borrow R, Cartwright K, Miller E. 2006. Immunogenicity and boosting after a reduced number of doses of a pneumococcal conjugate vaccine in infants and toddlers. Pediatr. Infect. Dis. J. 25:312–319 [DOI] [PubMed] [Google Scholar]
- 28. Huebner RE, Mbelle N, Forrest B, Madore DV, Klugman KP. 2004. Long-term antibody levels and booster responses in South African children immunized with nonavalent pneumococcal conjugate vaccine. Vaccine 22:2696–2700 [DOI] [PubMed] [Google Scholar]
- 29. Madhi SA, Adrian P, Kuwanda L, Jassat W, Jones S, Little T, Soininen A, Cutland C, Klugman KP. 2007. Long-term immunogenicity and efficacy of a 9-valent conjugate pneumococcal vaccine in human immunodeficient virus infected and non-infected children in the absence of a booster dose of vaccine. Vaccine 25:2451–2457 [DOI] [PubMed] [Google Scholar]
- 30. Schuerman L, Prymula R, Chrobok V, Dieussaert I, Poolman J. 2007. Kinetics of the immune response following pneumococcal PD conjugate vaccination. Vaccine 25:1953–1961 [DOI] [PubMed] [Google Scholar]
- 31. Silfverdal SA, Skerlikova H, Zanova M, Papuchova D, Traskine M, Borys D, Schuerman L. 2011. Anamnestic immune response in 3- to 4-year-old children previously immunized with 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine as 2-dose or 3-dose priming and a booster dose in the first year of life. Pediatr. Infect. Dis. J. 30:e155–e163 [DOI] [PubMed] [Google Scholar]
- 32. Madhi SA, Klugman KP, Kuwanda L, Cutland C, Käyhty H, Adrian P. 2009. Quantitative and qualitative anamnestic immune responses to pneumococcal conjugate vaccine in HIV-infected and HIV-uninfected children 5 years after vaccination. J. Infect. Dis. 199:1168–1176 [DOI] [PubMed] [Google Scholar]
- 33. Nurkka A, Åhman H, Yaich M, Eskola J, Käyhty H. 2001. Serum and salivary anti-capsular antibodies in infants and children vaccinated with octavalent pneumococcal conjugate vaccines, PncD and PncT. Vaccine 20:194–201 [DOI] [PubMed] [Google Scholar]
- 34. Åhman H, Käyhty Lehtonen H, Leroy H, Froeschle O, Jand Eskola J. 1998. Streptococcus pneumoniae capsular polysaccharide-diphtheria toxoid conjugate vaccine is immunogenic in early infancy and able to induce immunologic memory. Pediatr. Infect. Dis. J. 17:211–216 [DOI] [PubMed] [Google Scholar]