Figure 2. Hypothesized conformations of tau.
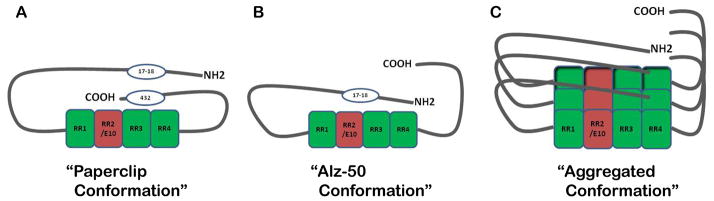
(A) The “paperclip conformation” was proposed using FRET analysis outlined by Jegenathan et al., 2006. The C-terminus folds in close proximity to the MTBRs while the N-terminus folds back towards the C-terminus but at a distance from the MTBRs. (B) The “Alz-50 conformation” is assumed when tau aggregation begins. When the C-terminus vacates its position near the MTBRs, it allows room for the N-terminus to interact with this region. This structure is recognized by the Alz-50 antibody and is associated with early tau polymerization as outlined by Carmel et al., 1996. (C) Putative stacking of tau monomers represents an aggregated tau conformation.
