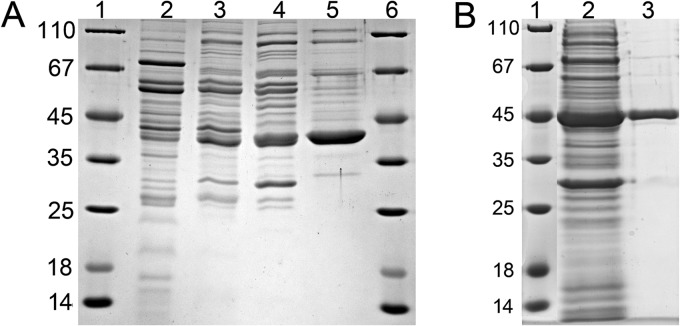
Fig 3.
Enrichment of ChCoA dehydrogenase. SDS-polyacrylamide gels of ChCoA-oxidizing activity (8 μg of protein per lane) are shown. (A) Purification from S. aciditrophicus. The protein band of about 40 kDa is highly enriched. Lanes 1 and 6, molecular mass standards; lane 2, cell extract; lane 3, fractions obtained after chromatography on octyl Sepharose; lane 4, hydroxyapatite; lane 5, MonoQ. (B) Purification after heterologous expression of its gene (SYN_02586) in E. coli BL21. Lane 1, molecular mass standard; lane 2, supernatant after ultracentrifugation; lane 3, pooled activity-containing fractions after Ni-chelating affinity chromatography.