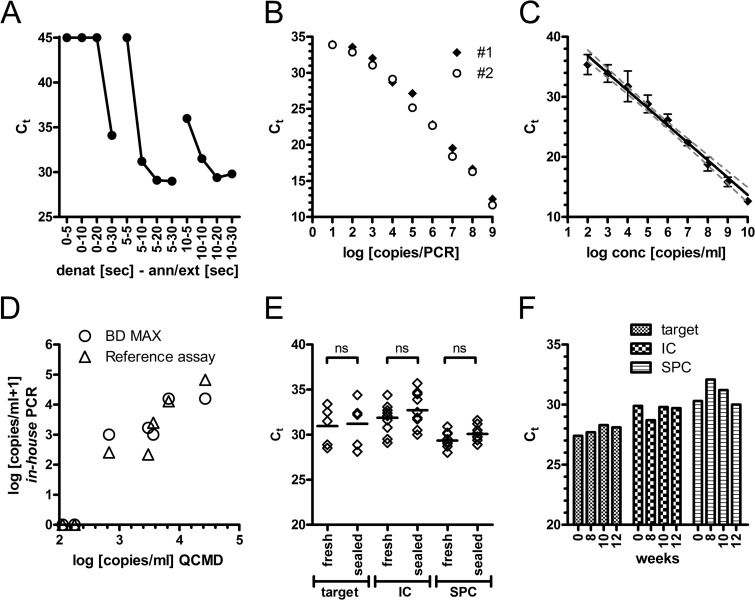
Fig 1.
Technical evaluation of the BD MAX PCR. (A) The BD MAX PCR was run in the PCR-only mode, and the indicated combinations of denaturation and annealing/extension times (two-step protocol) were tested with 6 log10 copies of a plasmid standard. (B) Serial dilutions of an MSG-encoding plasmid standard were evaluated in the PCR-only mode in duplicates. CT values of the MSG target gene amplification (FAM) are plotted against the plasmid copy numbers within the PCR. (C) A total of 200 μl of serial dilutions of the plasmid standard in three negative tested BAL fluid matrices was evaluated for automated DNA extraction and PCR amplification. Mean (± standard deviation [SD]) CT values of the MSG target gene amplification (FAM) are shown (n = 3). (D) The reference and BD MAX PCRs were used to evaluate the 2011 QCMD quality panel. Concentrations determined by use of a plasmid standard are plotted against the reference data. (E) PCR reagents were either tested immediately (fresh) or aliquoted, sealed, and stored overnight at −20°C. Ten patient samples (5 positive, 5 negative) were tested with both preparations. Median and individual CT data for the target MSG gene (FAM), internal control (Texas Red), and sample process control (Cy5) are shown. An unpaired t test showed no significant differences (ns). (F) A 5 log10 plasmid standard was tested repeatedly over 12 weeks with stored PCR reagents (target, MSG; IC, internal control; SPC, sample process control).