Abstract
Despite recent advances, tuberculosis (TB) diagnosis remains imperfect in resource-limited settings due to its complexity and costs, poor sensitivity of available tests, or long times to reporting. We present a report on the use of colorimetric methods, based on the detection of mycobacterial growth using colorimetric indicators, for the detection of Mycobacterium tuberculosis in sputum specimens. We evaluated the nitrate reductase assay (NRA), a modified NRA using para-nitrobenzoic acid (PNB) (NRAp), and the resazurin tube assay using PNB (RETAp) to differentiate tuberculous and nontuberculous mycobacteria. The performances were assessed at days 18 and 28 using mycobacterium growth indicator tube (MGIT) and Löwenstein-Jensen (LJ) medium culture methods as the reference standards. We enrolled 690 adults with suspected pulmonary tuberculosis from a regional referral hospital in Uganda between March 2010 and June 2011. At day 18, the sensitivities and specificities were 84.6% and 90.0% for the NRA, 84.1% and 92.6% for the NRAp, and 71.2% and 99.3% for the RETAp, respectively. At day 28, the sensitivity of the RETAp increased to 82.6%. Among smear-negative patients with suspected TB, sensitivities at day 28 were 64.7% for the NRA, 61.3% for the NRAp, and 50% for the RETAp. Contamination rates were found to be 5.4% for the NRA and 6.7% for the RETAp, compared with 22.1% for LJ medium culture and 20.4% for MGIT culture. The median times to positivity were 10, 7, and 25 days for colorimetric methods, MGIT culture, and LJ medium culture,respectively. Whereas the low specificity of the NRA/NRAp precludes it from being used for TB diagnosis, the RETAp might provide an alternative to LJ medium culture to decrease the time to culture results in resource-poor settings.
INTRODUCTION
Tuberculosis (TB) presents a large burden in developing countries, where its diagnosis relies mainly on smear microscopy (1). Although it is simple, fast, and inexpensive, microscopy has low and variable sensitivity (20 to 60%) (2), especially among patients coinfected with HIV (3).Culture is the reference standard but is not readily available in these countries. In addition, growth in the solid culture medium (Löwenstein-Jensen [LJ] medium) is too slow (3 to 8 weeks) to have an impact on patient management.
Liquid culture in a mycobacterium growth indicator tube (MGIT) is more rapid (10 days) and sensitive than culture in LJ medium but is costly and is more susceptible to contamination (4). Molecular approaches such as the Xpert MTB/RIF assay (5) reduce the time to detection (5, 6) and have been endorsed by the WHO (7, 8). Although they are sensitive, rapid, and relatively easy to use, these tests are costly and cannot completely replace culture, since they do not allow for the full range of drug sensitivity testing and Mycobacterium tuberculosis strain isolation (5, 9, 10).
Noncommercial alternative culture methods have been developed to reduce assay times and costs. Thin-layer agar (TLA) culture and microscopic observation drug susceptibility (MODS) assays (9, 10) have shown good sensitivity and specificity, compared with culture in LJ medium, and decreased time to detection (11.5 and 7 days, respectively). Both methods, however, require specific equipment in addition to the regular TB culture infrastructure, i.e., an inverted microscope for MODS assays and a CO2 incubator for TLA culture (9, 10). In addition, they require well-trained and experienced personnel for accurate reading.
Colorimetric methods currently are used for rapid identification and drug sensitivity testing of M. tuberculosis (11–15). These methods rely on the detection of live bacteria through enzymatic activity. Results are obtained by direct interpretation of the medium color and testing can be performed with minimal TB culture infrastructure, at relatively low cost (16).
The resazurin microtiter assay (REMA) relies on the ability of live bacteria to reduce the oxidation-reduction indicator resazurin in liquid medium (12, 17, 18). The nitrate reductase assay (NRA) is based on the ability of M. tuberculosis to reduce nitrate in solid medium (16). Drug sensitivity results can be obtained with the two methods in 7 days from isolates and in 14 days from sputum samples, with excellent sensitivity and specificity in comparison with the proportion method using LJ medium (15, 19, 20). REMA and NRA are endorsed by the WHO for rapid drug sensitivity testing from primary cultures for colorimetric redox indicator methods and from smear-positive sputum or primary cultures for NRA (8, 21). None of these methods has been evaluated for primary detection of M. tuberculosis in sputum specimens from patients with suspected TB.
In this study, we hypothesized that these methods might be applied for primary M. tuberculosis detection in sputum samples from patients with suspected pulmonary TB. For differentiation between M. tuberculosis and nontuberculous mycobacteria (NTM), we modified the resazurin tube assay (RETA) method with the addition of an identification step using a tube containing para-nitrobenzoic acid (PNB), which inhibits the growth of M. tuberculosis but not NTM (i.e., RETA with PNB [RETAp]). Since nitrate reduction is one of the biochemical tests used for the differentiation of M. tuberculosis and NTM, we evaluated the NRA method both with (NRAp) and without (NRA) the identification step using PNB.
The objective of this study was to evaluate the performance and feasibility of RETAp, NRA, and NRAp for the detection of M. tuberculosis complex (MTBC) from sputum in a setting with high prevalence rates of TB and HIV. We aimed to determine the performance of colorimetric assays in comparison with LJ medium culture and manual MGIT culture for all, HIV-positive, and smear-negative patients with suspected pulmonary TB.
MATERIALS AND METHODS
Participants.
We enrolled participants from the outpatient department and immune suppression syndrome (HIV) clinic at the Mbarara Regional Referral Hospital in southwestern Uganda from March 2010 through June 2011. Patients were eligible if they reported a cough for more than 2 weeks, were at least 15 years of age, and signed an informed-consent form. We excluded patients with grossly bloody sputum or clear saliva, those who could not produce at least 1 ml of sputum, and those who had received anti-TB treatment for at least 1 week in the month before enrollment.
The sample size of 690 patients was estimated based on the following hypotheses. We needed 96 culture-positive samples to estimate an expected sensitivity of 90% with a precision of 6%. With an expected proportion of 20% culture-positive pulmonary TB cases, we needed to enroll 480 patients. The sample size was increased to 690 to stratify the analysis for smear-negative patients with suspected TB, who represented 80% of the overall number of patients with suspected TB, and to take into account a potential dropout rate of 15% (22). The study protocol was approved by the Mbarara University Faculty of Medicine Research and Ethics Committee and Institutional Ethics Committee, the Uganda National Council for Science and Technology, and the Comité de Protection des Personnes, Ile de France XI, Saint-Germain en Laye, France.
Clinical assessment, chest radiography, and HIV testing.
All study participants underwent a physical assessment performed by a clinical officer and an anteroposterior chest X-ray. The study clinician recorded chest radiographic findings according to a predetermined pictorial tick-sheet, with final classification of the X-ray as “normal,” “abnormal, possible TB,” or “highly suggestive of TB.” Quality assurance consisted of paired interpretation of 10% of the X-rays by the hospital radiologist. HIV testing was performed for all patients who agreed, after they received pretest counseling and signed a separate informed-consent form. HIV testing was performed according to the national algorithm using Determine HIV-1/2/O (Abbott Laboratories, Abbott Park, IL) and HIV 1/2 Stat-Pak Ultra Fast (Chembio Diagnostic Systems, Medford, NY) tests, with the Uni-Gold recombinant HIV-1/2 (Trinity Biotech, Bray, Ireland) test for discordant results between the first two tests.
Tuberculosis laboratory procedures. (i) Microscopy and reference culture methods.
We collected sputum samples on the spot and the next morning. We used the light-emitting diode-auramine fluorescence technique for direct microscopy on each specimen (22–25) and reported the results according to the WHO grading scale (19). The reference standard and colorimetric methods were performed with the best-quality specimen according to the laboratory technician's assessment. The specimen was then decontaminated using the N-acetyl-l-cysteine (0.5%)-NaOH (1.5%) method (26).
For the reference culture methods, we inoculated 100 μl of decontaminated sputum into two homemade LJ medium tubes and 500 μl into one manual-testing MGIT (Becton, Dickinson, Franklin Lakes, NJ). We reported a negative culture result after 56 days of incubation. Liquefied or discolored LJ medium indicated contamination. Contamination from growth in an MGIT was ruled out using Ziehl-Neelsen (ZN) microscopy and culture on blood agar. For all positive LJ medium and MGIT cultures, we differentiated between M. tuberculosis and NTM using the SD TB Ag MPT64 Rapid system (SD Bioline, Kyongi-do, South Korea), following the manufacturer's instructions. The GenoType Mycobacterium CM/AS identification kit (Hain Lifescience, Nehren, Germany) was used for identification of NTM.
(ii) Colorimetric methods.
For the RETAp, we inoculated 100 μl of decontaminated sample into each of four 1.5-ml microtubes containing 500 μl of supplemented liquid medium (Middlebrook 7H9 medium supplemented with oleic acid, albumin, dextrose, and catalase, with 0.5% glycerol, 0.01% Casitone, and polymixin B-amphotericin B-nalidixic acid-trimethoprim-azlocillin [PANTA]) (17) and one microtube containing 500 μl of the same medium with PNB (500 μg/ml). After incubating the sample for 10 days at 37°C, we added 75 μl of resazurin solution (0.01%; Sigma-Aldrich, St. Louis, MO) to the first microtube and incubated the mixture overnight at 37°C. If a color change from blue (oxidized state) to pink (reduced state) occurred, indicating growth of the bacteria, then we inoculated one drop of the mixture on blood agar and we used one drop for ZN smear microscopy. If there was growth on blood agar and no acid-fast bacilli (AFB) were seen, then we reported the tube as contaminated. We considered the result to be indeterminate if there was no growth on blood agar and no AFB were seen or there was growth on blood agar and AFB were seen. All positive cultures (AFB with negative blood agar results) were identified as M. tuberculosis or NTM by treating the tube containing PNB with resazurin. A color change in the tube containing PNB was considered indicative of NTM, while no color change in the tube was considered indicative of M. tuberculosis. If there was no color change in the first tube at day 10, then the other tubes were tested on days 14, 18, and 28, using the same procedure. If there was no color change in the final tube (day 18 or day 28), then the sample was considered negative. If the final tube was contaminated, then the sample was considered to be contaminated.
For the NRA, we inoculated 200 μl of decontaminated sample into four tubes of LJ medium containing a final concentration of 1 g/liter of potassium nitrate (Sigma-Aldrich, St. Louis, MO) and one tube of LJ medium containing the same concentration of potassium nitrate with PNB (500 μg/ml). After incubating the samples for 10 days at 37°C, we added 500 μl of fresh reagent mixture (1 part 50% concentrated hydrochloric acid, 2 parts 0.2% sulfanilamide, and 2 parts 0.1% N-[1-naphthyl]ethylenediamine dihydrochloride) to the first tube. If the color of the reagent mixture changed to purple immediately after the addition of the reagent to this tube, we considered the result to be positive. For NRA interpretation, a positive result was considered positive for M. tuberculosis. For the NRAp, the NRA PNB tube was treated similarly; the test was considered positive for MTBC if there was no color change and positive for NTM if there was a color change. Culture results were interpreted in isolation from all other clinical and laboratory results.
(iii) Quality control and quality assessment.
We randomly selected 10% of all sputum smear slides and all slides with positive or scanty results that were read in duplicate for internal quality control. Discrepancies were resolved by the TB laboratory supervisor. Our laboratory participates in a proficiency scheme with the National Health Laboratory Services in South Africa for external quality control of TB cultures and smear microscopy.
Statistical analysis.
Data were double-entered using Voozanoo software (EpiConcept, Paris, France). Statistical analysis was performed using Stata SE v.11 software (StataCorp, College Station, TX).
We considered a sample positive by the standard method if MTBC was isolated from either LJ medium or an MGIT. We considered a sample negative if both results were negative or if one culture was negative and the other was contaminated. For each colorimetric assay, performance was calculated by estimating the sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios, with their 95% confidence intervals (CIs). Contaminated and positive culture results for NTM with either the reference standard or the tested culture methods were excluded from the analysis of performance. The performances of the NRA and RETAp were estimated separately after 18 and 28 days of incubation. Analysis was performed for all smear-negative and HIV-infected patients with suspected pulmonary TB. In a secondary analysis, we compared the M. tuberculosis recovery rates between the RETAp and LJ medium culture among MGIT-positive cases by using a McNemar test. We described the median times (with interquartile ranges [IQRs]) to culture positivity for the different culture methods.
For the feasibility assessment, laboratory technicians were asked, after the conclusion of the study, to complete an ease-of-use questionnaire on preparation of the culture media, processing of the specimens, and reading of the results. We calculated the costs of reagents and materials for the different methods.
RESULTS
Participants.
We screened 1,071 patients with suspected TB and included 671 patients (62.7%) in the final analysis (Fig. 1). Participant characteristics are presented in Table 1. Of these 671 patients, 590 patients (87.9%) provided two specimens. The specimen selected for the study was the early-morning specimen for 529 subjects (78.8%). The macroscopic appearance was purulent-mucopurulent for 212 samples (31.6%), mucoid for 446 (66.5%), blood-stained for 2 (0.3%), salivary for 2 (0.3%), and unknown for 9 (1.3%). We detected 108 patients (16.1%) with positive smear results among the 671 patients included in the analysis.
Fig 1.
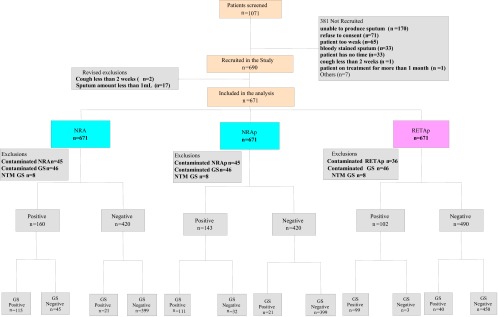
Recruitment of patients and culture results. GS, gold standard method.
Table 1.
Baseline characteristics of patients included in the study (n = 671 unless specified otherwise)
| Characteristica | Result |
|---|---|
| Median age (IQR) (yr) | 38 (30–48) |
| Gender ratio (male/female) | 49/51 |
| No. (%) of patients referred by: | |
| OPD | 392 (58.4) |
| ISS clinic | 257 (38.3) |
| Other | 22 (3.3) |
| No. (%) of HIV-positive patients (n = 637) | 373 (58.6) |
| No. (%) of patients with clinical signs at inclusion of: | |
| History of cough (n = 671) | 671 (100.0) |
| Fever for >2 wk (n = 670) | 258 (38.5) |
| Chest pain (n = 663) | 482 (72.7) |
| Hemoptysis (n = 664) | 78 (11.8) |
| Night sweats (n = 663) | 102 (15.4) |
| Reported weight loss (n = 663) | 488 (73.6) |
| No. (%) of patients (n = 654) with treatment history of: | |
| ≥1 medication in previous 30 days | 654 (100.0) |
| Antibiotics | 425 (65.0) |
| Cotrimoxazole | 329 (50.3) |
| Amoxicillin and derivatives | 140 (21.4) |
| Anti-TB drugs >1 mo before inclusion | 17 (2.6) |
| Previous antiretroviral treatment | 176 (26.9) |
| Antiretroviral treatment at inclusion | 81 (12.4) |
| No. (%) of patients (n = 611) with chest X-ray outcomes of: | |
| No TB | 259 (42.5) |
| Maybe TB | 215 (35.4) |
| Definitely TB | 135 (22.1) |
OPD, outpatient department; ISS, immune suppression syndrome.
Reference culture results.
Of the 671 patients included in the analysis, LJ medium culture results were M. tuberculosis positive for 107 (16.0%), NTM positive for 4 (0.06%), negative for 416 (62.0%), and contaminated for 148 (22.1%). MGIT results were M. tuberculosis positive for 142 patients (21.2%), NTM positive for 8 (1.2%), negative for 392 (58.4%), and contaminated for 137 (20.4%). When MGIT and LJ methods were combined, 46 (6.9%) and 8 (1.2%) patients had final contaminated culture and NTM results, respectively. After the exclusion of contaminated and NTM results, the reference standard results were positive for 148 patients (23.7%). The proportions of MTBC culture-positive results with the reference method were 6.2% (35/563 patients) among smear-negative patients and 21.8% (73/335 patients) among HIV-infected patients.
Colorimetric assay results.
The results of the NRA, NRAp, and RETAp after 18 days of incubation, cross-tabulated with the reference culture results, are shown in Table 2. At day 28, in comparison with day 18, an additional 19 MTBC cases were identified by the RETAp, for an incremental yield of 18.1% (19/105 patients), and 13 by the NRA or NRAp, for incremental yields of 8.7% (13/149 patients) for the NRA and 7.5% (13/174 patients) for the NRAp (Table 3). Accordingly, the sensitivities of the three methods increased between day 18 and day 28, while the specificities decreased slightly (Tables 4 and 5). Sensitivities were lower among smear-negative and HIV-infected patients than among all patients with suspected TB (Tables 4 and 5).
Table 2.
Results of colorimetric assays at day 18 according to reference standard results (n = 671)
| Colorimetric assay and result | No. with reference standard results of: |
|||
|---|---|---|---|---|
| Negative | MTBC | NTM | Contaminated | |
| NRA | ||||
| Negative | 399 | 21 | 6 | 26 |
| Positive | 45 | 115 | 1 | 13 |
| Contaminated | 33 | 4 | 1 | 7 |
| NRAp | ||||
| Negative | 399 | 21 | 6 | 26 |
| MTBC | 32 | 111 | 0 | 6 |
| NTM | 13 | 4 | 1 | 7 |
| Contaminated | 33 | 4 | 1 | 7 |
| RETAp | ||||
| Negative | 450 | 40 | 6 | 30 |
| MTBC | 3 | 99 | 0 | 0 |
| NTM | 3 | 0 | 1 | 0 |
| Indeterminate | 2 | 0 | 0 | 1 |
| Contaminated | 19 | 1 | 1 | 15 |
Table 3.
Results of colorimetric assays at day 28 according to reference standard results (n = 671)
| Colorimetric assay and result | No. with reference standard results of: |
|||
|---|---|---|---|---|
| Negative | MTBC | NTM | Contaminated | |
| NRA | ||||
| Negative | 392 | 19 | 6 | 24 |
| Positive | 53 | 118 | 1 | 15 |
| Contaminated | 32 | 3 | 1 | 7 |
| NRAp | ||||
| Negative | 392 | 19 | 6 | 24 |
| MTBC | 40 | 114 | 0 | 8 |
| NTM | 13 | 4 | 1 | 7 |
| Contaminated | 32 | 3 | 1 | 7 |
| RETAp | ||||
| Negative | 448 | 24 | 7 | 27 |
| MTBC | 5 | 114 | 0 | 2 |
| NTM | 3 | 0 | 1 | 0 |
| Indeterminate | 1 | 1 | 0 | 1 |
| Contaminated | 19 | 1 | 0 | 16 |
Table 4.
Performance of colorimetric methods for all, smear-negative, or HIV-positive patients with suspected TB at day 18a
| Assay and patient group | Sensitivity (% [95% CI]) | Specificity (% [95% CI]) | PPV (% [95% CI]) | NPV (% [95% CI]) | Positive LR (95% CI) | Negative LR (95% CI) |
|---|---|---|---|---|---|---|
| NRA | ||||||
| All | 84.6 (77.4–90.2) | 90.0 (86.7–92.5) | 71.9 (64.2–78.7) | 95.0 (92.5–96.9) | 8 (6–11) | 0.17 (0.12–0.25) |
| Smear negative | 61.8 (43.6–77.8) | 90.0 (86.9–92.7) | 32.3 (21.2–45.1) | 96.8 (94.7–98.3) | 6 (4–9) | 0.42 (0.28–0.65) |
| HIV positive | 78.1 (66.9–86.9) | 88.5 (84.0–92.1) | 65.5 (54.6–75.4) | 93.5 (89.7–96.3) | 7 (5–10) | 0.25 (0.16–0.38) |
| NRAp | ||||||
| All | 84.1 (76.7–89.9) | 92.6 (89.7–94.9) | 77.6 (69.9–84.2) | 95.0 (92.5–96.9) | 11 (8–16) | 0.17 (0.12–0.25) |
| Smear negative | 58.1 (39.1–75.5) | 92.8 (89.9–95.0) | 36.7 (23.4–51.7) | 96.8 (94.7–98.3) | 8 (5–12) | 0.45 (0.30–0.68) |
| HIV positive | 77.5 (66.0–86.5) | 91.7 (87.5–94.8) | 72.4 (60.9–82.0) | 93.5 (89.7–96.3) | 9 (6–14) | 0.25 (0.16–0.38) |
| RETAp | ||||||
| All | 71.2 (62.9–78.6) | 99.3 (98.1–99.9) | 97.1 (91.6–99.4) | 91.8 (89.0–94.1) | 107 (35–334) | 0.29 (0.22–0.38) |
| Smear negative | 25.7 (12.5–43.3) | 99.3 (98.1–99.9) | 75.0 (42.8–94.5) | 94.5 (92.1–96.4) | 39 (11–136) | 0.75 (0.62–0.91) |
| HIV positive | 63.0 (50.9–74.0) | 98.8 (96.6–99.8) | 93.9 (83.1–98.7) | 90.4 (86.3–93.5) | 54 (17–168) | 0.37 (0.28–0.51) |
PPV, positive predictive value; NPV, negative predictive value; LR, likelihood ratio.
Table 5.
Performance of colorimetric methods for all, smear-negative, or HIV-positive patients at day 28
| Assay and patient group | Sensitivity (% [95% CI]) | Specificity (% [95% CI]) | PPVa (% [95% CI]) | NPV (% [95% CI]) | Positive LR (95% CI) | Negative LR (95% CI) |
|---|---|---|---|---|---|---|
| NRA | ||||||
| All | 86.1 (79.2–91.4) | 88.1 (84.7–90.9) | 69.0 (61.5–75.8) | 95.4 (92.9–97.2) | 7 (6–9) | 0.16 (0.10–0.24) |
| Smear negative | 64.7 (46.5–80.3) | 88.3 (84.8–91.1) | 29.7 (19.7–41.5) | 97.0 (94.9–98.5) | 6 (4–8) | 0.40 (0.25–0.63) |
| HIV positive | 78.1 (66.9–86.9) | 88.5 (84.0–92.1) | 65.5 (54.6–75.4) | 93.5 (89.7–96.3) | 7 (5–10) | 0.25 (0.16–0.38) |
| NRAp | ||||||
| All | 85.7 (78.6–91.2) | 90.7 (87.6–93.2) | 74.0 (66.4–80.8) | 95.4 (92.9–97.2) | 9 (7–13) | 0.16 (0.10–0.24) |
| Smear negative | 61.3 (42.2–78.2) | 90.9 (87.8–93.5) | 32.8 (21.0–46.3) | 97.0 (94.9–98.5) | 7 (4–10) | 0.43 (0.27–0.66) |
| HIV positive | 80.3 (69.1–88.8) | 89.3 (84.9–92.8) | 67.9 (56.8–77.6)) | 94.2 (90.4–96.8) | 8 (5–11) | 0.22 (0.14–0.35) |
| RETAp | ||||||
| All | 82.6 (75.2–88.5) | 98.9 (97.4–99.6) | 95.8 (90.5–98.6) | 94.9 (93.5–96.7) | 75 (31–180) | 0.18 (0.12–0.25) |
| Smear negative | 50.0 (32.4–67.6) | 98.9 (97.4–99.6) | 77.3 (54.6–92.2) | 96.3 (94.2–97.8) | 45 (17–115) | 0.51 (0.36–0.71) |
| HIV positive | 77.8 (66.4–86.7) | 98.0 (95.5–99.4) | 91.8 (81.9–97.3) | 94.0 (90.5–96.5) | 40 (17–96) | 0.23 (0.15–0.35) |
PPV, positive predictive value; NPV, negative predictive value; LR, likelihood ratio.
There were 53 false-positive results with the NRA methods, among which the NRAp classified 13 cases as NTM. Among the 40 cases classified as MTBC by the NRAp, chest radiographic results were classified as possible or highly suggestive of TB in 45.5% (15/33 cases); 73.0% of patients (27/37 patients) were HIV positive, and one was smear positive (2.5%). Results of both reference culture methods were negative for 19 of the false-positive NRAp specimens (47.5%), while one result was negative and the other contaminated for the remaining 21 specimens (52.5%). Of the 142 specimens that were positive by MGIT culture, 101 (71%) were positive by LJ medium culture and 111 (78%) by the RETAp at day 28 (P = 0.099); however, there were 20 (14%) contaminated results for LJ medium culture, compared with 0 (0%) for the RETAp.
Time to positivity.
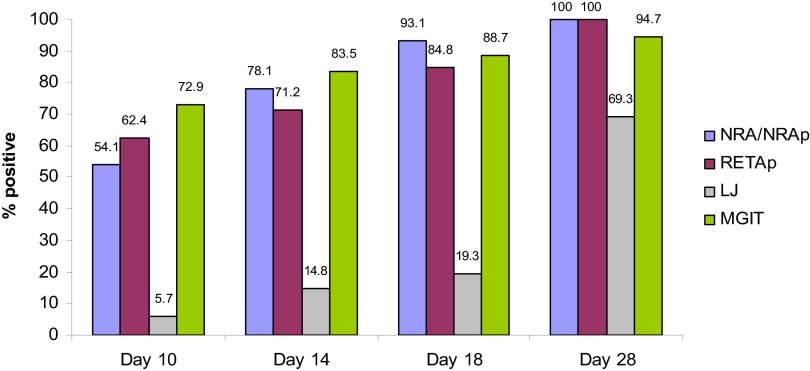
The median times to positivity were 10 days (IQR, 10 to 14 days) for the NRA, 10 days (IQR, 10 to 14 days) for the NRAp, and 10 days (IQR, 10 to 18 days) for the RETAp. In comparison, the median times to positivity were 7 days (IQR, 4 to 11 days) for MGIT culture and 25 days (IQR, 21 to 35 days) for LJ medium culture. The NRA/NRAp detected 93.1% of positive culture specimens and the RETAp detected 84.8% by day 18 of incubation (Fig. 2).
Fig 2.
Cumulative percentages of positive specimens on days 10, 14, 18, and 28 for each medium.
Feasibility and cost.
Each medium was prepared locally, was stored at room temperature, and cost less than $1 for the RETAp or NRA. The analysis of one sample cost $0.5 and $10 for LJ medium culture and MGIT culture, respectively. Laboratory technicians reported high acceptability and feasibility for the colorimetric assays.
DISCUSSION
We present the first report on the use of colorimetric methods for primary detection of M. tuberculosis for the diagnosis of pulmonary TB. These assays were simple to use and showed low contamination levels with relatively short times to positive results, compared with standard culture methods; however, they had disappointing performances.
The NRA method showed acceptable sensitivity overall (84.6%) but had low specificity (90%). The addition of PNB in the NRAp method slightly increased the specificity to 93%. The low specificity might be due to false-positive results caused by contaminants that can cause nitrate reduction, such as Pseudomonas spp. or Staphylococcus spp. (27, 28). This might partly explain the lower contamination rate observed for the NRA (6.7%) than for LJ medium culture (22.1%). An additional step of ZN microscopy and/or M. tuberculosis identification rapid testing for positive samples might improve NRA specificity.
In contrast, the RETAp had excellent specificity (99.3%) but its sensitivity (71.2%) was relatively low at day 18, especially among smear-negative patients (50%). Extending detection to 28 days instead of 18 days increased the sensitivity to 82.6%, and an additional reading after 28 days should be systematically planned for negative RETAp samples.
The performances of the colorimetric methods for primary M. tuberculosis detection seem to be slightly lower than the performances of MODS testing and TLA culture from a recent systematic review, with sensitivities of 92% (95% CI, 87 to 97%) for MODS assays and 87% (95% CI, 79 to 94%) for TLA culture and specificities of 96% (95% CI, 90 to 100%) for MODS assays and 98% (95% CI, 94 to 100%) for TLA culture (9). However, the performances of RETAp after 28 days are comparable to those of MODS assays in studies including both smear-positive and smear-negative specimens and using liquid and solid cultures as reference standards (9, 10). Nevertheless, compared with these other noncommercial assays, the colorimetric methods provide important advantages in terms of ease of interpretation, because they rely on a color change instead of observation of microcolonies under the microscope (10, 29, 30) and thus probably are much less prone to interuser variability. Comparisons of accuracy, interuser reproducibility, and time to detection between these noncommercial rapid cultures would be helpful.
LJ medium culture remains the most-used culture method for diagnosing TB in developing countries, although it is less sensitive than MGIT culture (31). In this first study on colorimetric methods for M. tuberculosis detection, RETAp M. tuberculosis recovery results seem to be comparable to LJ medium culture results. However, the positive detection rate for LJ medium culture might be underestimated because of the large proportion of contaminated results for LJ medium culture, which represented 14.2% of the positive results with MGIT culture and 0% with the RETAp. The lower rate of contamination for the RETAp might be due to the addition of PANTA in the tube as well as the use of four tubes for RETAp detection, compared with two tubes for LJ medium culture. These results should be confirmed in another study but they are encouraging, taking into consideration the short time to positivity of the RETAp, compared with LJ medium culture (median, 10 days versus 25 days).
The performances of RETAp at day 28 are slightly lower than performances of the Xpert MTB/RIF test reported in numerous studies on this new method (pooled sensitivity values of 88% [95% CI, 83 to 92%] overall, 68% [95% CI, 59 to 75%] among smear-negative patients, and 76% [95% CI, 63 to 85%] among HIV-positive patients in reference 32). Culture methods in general cannot compare with this molecular test in terms of the time to obtain results and the simplicity of use. The main advantages of culture methods over the Xpert assay are that they can test for susceptibility to multiple drugs, as opposed to rifampin alone (6), and they allow the storage of cultured strains for quality control and further investigations. Culture methods also are more affordable for settings with existing culture-testing capacity; the Xpert MTB/RIF assay costs $10, compared with the homemade colorimetric media (less than $1 per specimen). However, in contrast to the Xpert MTB/RIF assay, all other culture methods require P2/P3 levels of safety equipment for the culture of M. tuberculosis.
Our study was limited by the lower-than-expected number of smear-negative and culture-positive specimens in our sample, which resulted in wide confidence intervals for analyses in this subgroup. This was due to overestimation of the M. tuberculosis growth in specimens from smear-negative patients with suspected pulmonary TB, based on a previous report of 10 to 20% in the region (22, 24, 25). Another limitation was the high contamination rates for LJ and MGIT media. Despite the high quality of the standard laboratory procedures, as confirmed by external monitors from the supranational reference laboratory at the Institute of Tropical Medicine (Antwerp, Belgium), contamination rates remained high (22%) throughout the study. A possible explanation is that our broad inclusion criterion, defined as cough lasting more than 2 weeks, might have led to inclusion of patients with diseases other than TB, caused by pathogens such as Pseudomonas spp. or Staphylococcus spp., which are known to be resistant to our decontamination process.
In conclusion, this first evaluation of colorimetric methods as noncommercial alternatives to MGIT culture for rapid M. tuberculosis culture-based detection shows disappointing results in terms of performance. The low specificity of the NRA, even after the addition of PNB, precludes it from being used for primary M. tuberculosis detection. However, the RETAp might have a place in laboratories already using LJ medium culture, to speed up culture results and potentially to reduce culture contamination. In addition, the ease of reading of the results is an advantage compared with other noncommercial culture methods. Further evaluation of the RETAp for diagnosis of TB in comparison with other noncommercial culture methods would be necessary.
ACKNOWLEDGMENTS
We thank Augusto Llosa, Mark Siedner, Family Health International (FHI), Mario Chan, Laura Philips, Kenneth Schultz, Suzanne Fisher, Juliet Mwanga-Amumpaire, Dan Nyehangane, and Kennedy Kassaza for their support in the preparation of the manuscript.
Footnotes
Published ahead of print 8 May 2013
REFERENCES
- 1. Perkins MD. 2000. New diagnostic tools for tuberculosis. Int. J. Tuberc. Lung Dis. 4:S182–S188 [PubMed] [Google Scholar]
- 2. Steingart KR, Ng V, Henry M, Hopewell PC, Ramsay A, Cunningham J, Urbanczik R, Perkins MD, Aziz MA, Pai M. 2006. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect. Dis. 6:664–674 [DOI] [PubMed] [Google Scholar]
- 3. Colebunders R, Bastian I. 2000. A review of the diagnosis and treatment of smear-negative pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 4:97–107 [PubMed] [Google Scholar]
- 4. Thornton CG, MacLellan KM, Brink TL, Jr, Passen S. 1998. In vitro comparison of NALC-NaOH, Tween 80, and C18-carboxypropylbetaine for processing of specimens for recovery of mycobacteria. J. Clin. Microbiol. 36:3558–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Theron G, Peter J, van Zyl-Smit R, Mishra H, Streicher E, Murray S, Dawson R, Whitelaw A, Hoelscher M, Sharma S, Pai M, Warren R, Dheda K. 2011. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am. J. Respir. Crit. Care Med. 184:132–140 [DOI] [PubMed] [Google Scholar]
- 6. Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O'Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N. Engl. J. Med. 363:1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization 2007. Strategic and Technical Advisory Group for Tuberculosis: report on conclusions and recommendations. World Health Organization, Geneva, Switzerland [Google Scholar]
- 8. World Health Organization 2010. Strategic and Technical Advisory Group for Tuberculosis: report on conclusions and recommendations. World Health Organization, Geneva, Switzerland [Google Scholar]
- 9. Leung E, Minion J, Benedetti A, Pai M, Menzies D. 2012. Microcolony culture techniques for tuberculosis diagnosis: a systematic review. Int. J. Tuberc. Lung Dis. 16:16–23 [DOI] [PubMed] [Google Scholar]
- 10. Moore DA, Evans CA, Gilman RH, Caviedes L, Coronel J, Vivar A, Sanchez E, Pinedo Y, Saravia JC, Salazar C, Oberhelman R, Hollm-Delgado MG, LaChira D, Escombe AR, Friedland JS. 2006. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N. Engl. J. Med. 355:1539–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Affolabi D, Odoun M, Sanoussi N, Martin A, Palomino JC, Kestens L, Anagonou S, Portaels F. 2008. Rapid and inexpensive detection of multidrug-resistant Mycobacterium tuberculosis with the nitrate reductase assay using liquid medium and direct application to sputum samples. J. Clin. Microbiol. 46:3243–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martin A, Camacho M, Portaels F, Palomino JC. 2003. Resazurin microtiter assay plate testing of Mycobacterium tuberculosis susceptibilities to second-line drugs: rapid, simple, and inexpensive method. Antimicrob. Agents Chemother. 47:3616–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martin A, Morcillo N, Lemus D, Montoro E, Telles MA, Simboli N, Pontino M, Porras T, Leon C, Velasco M, Chacon L, Barrera L, Ritacco V, Portaels F, Palomino JC. 2005. Multicenter study of MTT and resazurin assays for testing susceptibility to first-line anti-tuberculosis drugs. Int. J. Tuberc. Lung Dis. 9:901–906 [PubMed] [Google Scholar]
- 14. Martin A, Portaels F, Palomino JC. 2007. Colorimetric redox-indicator methods for the rapid detection of multidrug resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. J. Antimicrob. Chemother. 59:175–183 [DOI] [PubMed] [Google Scholar]
- 15. Palomino JC, Martin A, Portaels F. 2007. Rapid drug resistance detection in Mycobacterium tuberculosis: a review of colourimetric methods. Clin. Microbiol. Infect. 13:754–762 [DOI] [PubMed] [Google Scholar]
- 16. Angeby KA, Klintz L, Hoffner SE. 2002. Rapid and inexpensive drug susceptibility testing of Mycobacterium tuberculosis with a nitrate reductase assay. J. Clin. Microbiol. 40:553–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Palomino JC, Martin A, Camacho M, Guerra H, Swings J, Portaels F. 2002. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 46:2720–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palomino JC, Martin A, Portaels F. 2004. A microplate indicator-based method for determining the susceptibility of multidrug-resistant Mycobacterium tuberculosis to antimicrobial agents. Int. J. Tuberc. Lung Dis. 8:1510–1511 [PubMed] [Google Scholar]
- 19. Affolabi D, Odoun M, Martin A, Palomino JC, Anagonou S, Portaels F. 2007. Evaluation of direct detection of Mycobacterium tuberculosis rifampin resistance by a nitrate reductase assay applied to sputum samples in Cotonou, Benin. J. Clin. Microbiol. 45:2123–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin A, Montoro E, Lemus D, Simboli N, Morcillo N, Velasco M, Chauca J, Barrera L, Ritacco V, Portaels F, Palomino JC. 2005. Multicenter evaluation of the nitrate reductase assay for drug resistance detection of Mycobacterium tuberculosis. J. Microbiol. Methods 63:145–150 [DOI] [PubMed] [Google Scholar]
- 21. World Health Organization 2009. Strategic and Technical Advisory Group for Tuberculosis: report on conclusions and recommendations. World Health Organization, Geneva, Switzerland [Google Scholar]
- 22. Bonnet M, Gagnidze L, Varaine F, Ramsay A, Githui W, Guerin PJ. 2009. Evaluation of FASTPlaqueTB to diagnose smear-negative tuberculosis in a peripheral clinic in Kenya. Int. J. Tuberc. Lung Dis. 13:1112–1118 [PubMed] [Google Scholar]
- 23. Affolabi D, Torrea G, Odoun M, Senou N, Ali Ligali M, Anagonou S, Van Deun A. 2010. Comparison of two LED fluorescence microscopy build-on modules for acid-fast smear microscopy. Int. J. Tuberc. Lung Dis. 14:160–164 [PubMed] [Google Scholar]
- 24. Bonnet M, Gagnidze L, Githui W, Guerin PJ, Bonte L, Varaine F, Ramsay A. 2011. Performance of LED-based fluorescence microscopy to diagnose tuberculosis in a peripheral health centre in Nairobi. PLoS One 6:e17214. 10.1371/journal.pone.0017214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bonnet M, Gagnidze L, Guerin PJ, Bonte L, Ramsay A, Githui W, Varaine F. 2011. Evaluation of combined LED-fluorescence microscopy and bleach sedimentation for diagnosis of tuberculosis at peripheral health service level. PLoS One 6:e20175. 10.1371/journal.pone.0020175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kent PT, Kubica GP. 1985. Public health mycobacteriology: a guide for the level III laboratory, p 31–70 U.S. Department of Health and Human Services, Atlanta, GA [Google Scholar]
- 27. Coban AY. 2012. Rapid determination of methicillin resistance among Staphylococcus aureus clinical isolates by colorimetric methods. J. Clin. Microbiol. 50:2191–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sias SR, Stouthamer AH, Ingraham JL. 1980. The assimilatory and dissimilatory nitrate reductases of Pseudomonas aeruginosa are encoded by different genes. J. Gen. Microbiol. 118:229–234 [DOI] [PubMed] [Google Scholar]
- 29. Moore DA. 2007. Future prospects for the MODS assay in multidrug-resistant tuberculosis diagnosis. Future Microbiol. 2:97–101 [DOI] [PubMed] [Google Scholar]
- 30. Moore DA, Mendoza D, Gilman RH, Evans CA, Hollm Delgado MG, Guerra J, Caviedes L, Vargas D, Ticona E, Ortiz J, Soto G, Serpa J. 2004. Microscopic observation drug susceptibility assay, a rapid, reliable diagnostic test for multidrug-resistant tuberculosis suitable for use in resource-poor settings. J. Clin. Microbiol. 42:4432–4437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bwanga F, Haile M, Joloba ML, Ochom E, Hoffner S. 2011. Direct nitrate reductase assay versus microscopic observation drug susceptibility test for rapid detection of MDR-TB in Uganda. PLoS One 6:e19565. 10.1371/journal.pone.0019565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cohen D, Corbett E. 2013. Evidence supports TB test, so what now? Cochrane Database Syst. Rev. 2:ED000051. 10.1002/14651858.ED000051 [DOI] [PMC free article] [PubMed] [Google Scholar]