Abstract
Objective
Co-infection with hepatitis C virus (HCV) is a major cause of morbidity and mortality in HIV-infected individuals. However, predictors of mortality are poorly defined and most studies have focused predominantly on co-infection in men. We evaluated whether two indirect markers of hepatic fibrosis, aspartate aminotransferase-to-platelet ratio index (APRI) and FIB-4 scores, were predictive of mortality in a well defined longitudinal cohort of HCV/HIV-co-infected women on HAART.
Methods
HCV/HIV-co-infected women on antiretroviral therapy enrolled in Women’s Interagency HIV Study (WIHS), a National Institutes of Health-funded prospective, multicenter, cohort study of women with and at risk for HIV infection were included. Using Cox regression analysis, associations between APRI and FIB-4 with all-cause mortality were assessed.
Results
Four hundred and fifty HCV/HIV-co-infected women, of whom 191 women died, had a median follow-up of 6.6 years and 5739 WIHS visits. Compared with women with low APRI or FIB-4 levels, severe fibrosis was significantly associated with an increased risk of all-cause mortality {APRI: hazard ratio 2.78 [95% confidence interval (CI) 1.87, 4.12]; FIB-4: hazard ratio 2.58 (95% CI 1.68, 3.95)}. Crude death rates per 1000 patient-years increased with increasing liver fibrosis: 34.8 for mild, 51.3 for moderate and 167.9 for severe fibrosis as measured by FIB-4. Importantly, both APRI and FIB-4 increased during the 5 years prior to death for all women: the slope of increase was greater for women dying a liver-related death compared with nonliver-related death.
Conclusion
Both APRI and FIB-4 are independently associated with all-cause mortality in HCV/HIV-co-infected women and may have clinical prognostic utility among women with HIV and HCV.
Keywords: fibrosis markers, hepatitis C virus, HIV, longitudinal study, mortality
Introduction
Hepatitis C virus (HCV) co-infection is common in HIV-seropositive patients due to shared routes of viral transmission [1]. Compared with patients infected with HCV alone, individuals co-infected with HCV and HIV have accelerated liver disease progression [2,3]. Women with HCV have less liver disease progression than men [4]. With HIV-seropositive patients now living longer through use of HAART, HCV-related hepatic fibrosis and cirrhosis has become a major health problem. Liver disease is currently the leading cause of non-AIDS related death among HCV/HIV-co-infected patients [5].
Little is known about the predictors of mortality in HCV/HIV-co-infected individuals, and most of the data have been obtained in men [6,7]. As HCV-mono-infected women generally have a less aggressive course of liver disease than men, separate studies of women and men are necessary [4]. Assessment of liver disease severity, most notably the degree of hepatic fibrosis, is critical to guide treatment and prognostic counseling. Currently, liver biopsy is the gold standard for determining the degree of hepatic fibrosis; however, liver biopsy is also an invasive and costly procedure. Therefore, substantial efforts have been focused on the identification of noninvasive serum markers that are not only useful for identifying fibrosis among patients with chronic liver disease but are also easily obtained in the clinical setting. Two fibrosis marker scoring systems, the aspartate aminotransferase (AST)-to-platelet ratio index (APRI) [8] and FIB-4 index [based upon age, AST, alanine aminotransferase (ALT) level and platelet count] [9], have been developed. The aim of the current investigation was to assess the value of APRI and FIB-4 as markers of survival outcome among a large cohort of HCV/HIV-co-infected women. We chose these two markers as they are based upon readily available, routine laboratory tests and have been shown to be useful for predicting significant fibrosis among patients with HCV.
Materials and methods
Patient population
An analysis of clinical and laboratory parameters was performed in women enrolled in the Women’s Interagency HIV Study (WIHS). The details of this prospective, multicenter, longitudinal cohort study have been published [10]. Briefly, the WIHS enrolled 3 766 adult women in two cohorts (1994–1995 and 2001–2002) at six clinical sites across the United States who were either infected with HIV or at high risk for acquiring HIV. Overall, 32% of HIV-seropositive women were co-infected with HCV, acquired prior to enrolment in WIHS. Only 17 (1.8%) women received therapy for HCV. Overall participant retention and study follow-up was 82% as described [11]. All women gave written, informed consent for participation in WIHS. The WIHS study was approved by all Institutional Review Boards of the participating medical centers, and this study was reviewed and approved by the University of California San Francisco Institutional Review Board.
Because the advent of HAART fundamentally changed the natural history of HIV, this analysis was restricted to HCV/HIV-co-infected women on HAART. The first visit on HAART with available data for APRI and FIB-4 assessment is referred to as the index visit. In order to focus on chronic liver disease and minimize the possibility of including women with acute hepatic inflammation and severe thrombocytopenia resulting from causes other than HCV-related cirrhosis, we excluded study visits with AST or ALT greater than 10 times upper limit of normal (ULN) and platelet counts less than 25 000 × 106 cells/l. Only women with at least one follow-up visit after the index visit with laboratory and clinical values for calculation of APRI (AST and platelet count) and FIB-4 (AST, ALT, platelet count and age) were included in this study. Because of the limited number of women (n = 42) under 35 years of age at entry into WIHS, we included only those women who were at least 35 years of age at the index visit.
Data collection
Every 6 months, participants undergo a comprehensive physical examination, provide biological specimens for CD4 cell count and HIV-RNA viral load determination, and complete an interviewer-administered questionnaire that collects information on demographics, health history and medication use. Data on current alcohol usage were derived from a standardized questionnaire and categorized as light (<3 drinks/week), moderate (3–13 drinks/week) or heavy (>13 drinks per week) as well as number of drink per year over the time of the study. Intravenous drug use was also documented at each clinical visit. Hepatitis B virus status was assessed via testing for the surface antigen (HBsAg) within the first year of entry into WIHS. HCV infection was documented by testing for antibody to HCV by second-generation or third-generation enzyme linked immunoassay (Ortho-Diagnostic Systems, Rochester, New York, USA) and testing for the presence of HCV-RNA by HCV-branched DNA (Quantiplex 2.0 branched chain DNA-enhanced label amplification assay; Bayer-Versant Diagnostics, formerly Chiron Diagnostics, Emeryville, California, USA) and by reverse transcriptase polymerase chain reaction (COBAS Amplicor HCV Detection Kit, Roche Diagnostic Systems, Pleasanton, California, USA). HIV-RNA was measured using the isothermal nucleic acid sequence-based amplification (NASBA/Nuclisens) method (bio-Merieaux, Durham, North Carolina, USA) with a detection limit of 80 copies/ml.
The definition of HAART was based on the US Department of Health and Human Services (DHHS) treatment guidelines (www.aidsinfo.gov) as use of more than two nucleoside reverse transcriptase inhibitors (NRTIs) in combination with at least one protease inhibitor or one non-NRTI (NNRTI); one NRTI in combination with at least one protease inhibitor and at least one NNRTI; or an abacavir-containing or tenofovir-containing regimen of more than three NRTIs in the absence of both protease inhibitors and NNRTIs, except for the three NRTI regimens consisting of abacavir with tenofovir and lamivudine or didanosine with tenofovir and lamivudine.
Assessment of liver fibrosis
Two indirect markers of liver fibrosis were used for this analysis: APRI [8] and FIB-4 [9] with ALT ULN designated as 40 U/l. These measures can be calculated using readily available patient and laboratory data [AST, ALT, platelet count (× 109 cells/l) and age] according to the equations given below:
Assessment of mortality outcome
The primary outcome of interest for these analyses was all-cause mortality. A secondary outcome was cause of death, categorized as liver-related or nonliver-related, based on death certificate reporting of primary cause of death [12]. In order to detect all deaths among WIHS participants, a number of active and passive ascertainment methods were employed. Death certificates were obtained from medical records and local health departments as soon as the study staff became aware of a death. To assure that all deaths in the United States were ascertained, National Death Index (NDI)-Plus searches were performed annually for all WIHS participants who were known to have died or were lost to study follow-up. The NDI-Plus provides information on deaths that occur throughout the United States and US territories and provides all the primary and underlying causes from the original death certificates. All death certificate data were reviewed independently by two clinicians using specific criteria that classified a death as AIDS-related if an AIDS-defining infection or malignancy was the cause of death, or if the cause of death was pneumonia or sepsis in the setting of a recent CD4+ cell counts less than 200 cells/µl. Deaths were classified as indeterminate if the cause of death was entirely nonspecific (most frequently ‘cardiopulmonary arrest’), if the death certificate had conflicting causes or had HIV as the only cause of death for a woman whose CD4+ cell count was at least 200 cells/µl at the most recent WIHS visit. Deaths were classified as non-AIDS if a non-AIDS cause was the primary cause of death. Patients were followed up until death, the end of the follow-up period in December 2007 or until the last completed study visit.
Statistical analysis
Continuous data were summarized using median and interquartile range (IQR), and categorical variables were summarized using frequency and percentage. The role of APRI and FIB-4 fibrosis markers as predictors of all-cause mortality was assessed using semiparametric Cox proportional hazard regression, after adjusting for known predictors of post-HAART mortality, including pre-HAART peak HIV viral load (copies/ml), pre-HAART nadir CD4 count (cells/µl) and evidence of clinical AIDS pre-HAART. Pre-HAART nadir CD4 was a categorical variable based upon CD4 cut-points (<100, 100–200, 200–350, >350 cells/µl). Specifically, different models were constructed for APRI and FIB-4 in which each fibrosis marker was expressed as either a continuous (log-transformed) or categorical (mild, moderate, severe) variable. APRI and FIB-4 marker levels were treated as time-dependent with the most current (last available) used for these analyses. The cut-points used to categorize fibrosis severity were derived from previous studies of these markers and were as follows: APRI (<0.5 mild, 0.5–1.5 moderate, >1.5 severe) and FIB-4 (<1.5 mild, 1.5–3.25 moderate, >3.25 severe) [8,9]. As the date of HCV infection was unknown, and the risk of mortality is fundamentally associated with age, we utilized age as the timescale for the analysis in conjunction with left truncation (staggered entry) methods. Individuals contributed at-risk time to the analysis beginning at their age at the index visit.
In addition to modeling all-cause mortality, deaths were also classified as liver-related and nonliver-related for the subgroup of women who had at least two fibrosis marker measurements within the 5 years prior to death. Although the total number of liver-related deaths was not sufficient for a multivariate survival analysis (n = 27), longitudinal profiles of APRI and FIB-4 marker levels were compared among women with liver-related and nonliver-related deaths. For these analyses, linear random effects regression modeling was conducted to compare the liver-related and nonliver-related death groups, with time measured as years from death, and log10 transformation of fibrosis marker levels to make the data approximately normally distributed. To account for any nonlinearity, the model also included a smooth trend with time, which was allowed to vary between the liver-related and nonliver-related death groups. To estimate trends, spline models were utilized [13]. All data analyses were performed using SAS (SAS Institute Inc., Cary, North Carolina, USA) and S-Plus (Insightful Corp., Seattle, Washington, USA).
Results
Patient population
There were 450 HCV/HIV-co-infected women over 35 years of age who had at least one liver fibrosis marker (APRI or FIB-4) measurement post-HAART initiation (Table 1). Of these, 191 women died during follow-up. The characteristics of the study population at index visit, including those who died and those who survived are shown in Table 1. The median age of the women at index visit was 43 years (IQR 40–47 years). Two-thirds (62%) of the participants were non-Hispanic black (n = 281) and 20% (n = 92) were Hispanic. The majority of women reported abstinence from alcohol at their index visit; however, 18% were moderate-to-heavy drinkers. Only 17 (1.8)% of women were treated for HCV and 5% had diabetes. The 450 women included in these analyses participated in a total of 5739 WIHS visits. Median follow-up time was 6.6 years (IQR 3.2–9.8 years).
Table 1.
Characteristics of 450 HIV-positive, hepatitis C virus-positive women in Women’s Interagency HIV Study on HAART at index visit.
| Characteristicsa | Died (N = 191) | Alive at last follow-up (N = 259) | Overall (N = 450) |
|---|---|---|---|
| Age (years) | 44 (40–48) | 43 (39–47) | 43 (40–47) |
| Race/ethnicity [% (n)] | |||
| Non-Hispanic white | 15 (28) | 17 (43) | 16 (71) |
| Non-Hispanic black | 63 (121) | 62 (160) | 62 (281) |
| Hispanic | 20 (39) | 20 (53) | 20 (92) |
| Other | 2 (3) | 1 (3) | 1 (6) |
| Pre-HAART HIV peak viral load (copies/ml) | 93 000 (23 000–300 000) | 47 000 (9900–140 000) | 62000 (13 000–210 000) |
| Pre-HAART nadir CD4 cell counts (cells/µl) | 160 (81–272) | 241 (138–345) | 205 (102–316) |
| Clinical AIDS pre-HAART initiation [% (n)] | 61 (116) | 42 (110) | 50 (226) |
| Pre-HAART ART use [% (n)] | 79 (151) | 66 (170) | 71 (321) |
| HIV viral load (copies/ml) | 2250 (81–34 000) | 173 (80–3600) | 570 (80–13 000) |
| CD4 cell counts (cells/µl) | 230 (128–375) | 351 (200–516) | 313 (169–469) |
| HCV-RNA, ×103 IU/ml at entry into WIHS | 2880 (926–4960) | 1760 (551–3760) | 2085 (645–4020) |
| HBsAg-positive, % (n) at entry into WIHS | 3 (5) | 2 (4) | 2 (9) |
| Current alcohol use [% (n)] | |||
| Abstinent | 60 (115) | 59 (152) | 59 (267) |
| Light (<3 drinks/week) | 15 (28) | 24 (63) | 20 (91) |
| Moderate (3–13 drinks/week) | 16 (31) | 11 (29) | 1% (60) |
| Heavy (>13 drinks/week) | 7 (13) | 4 (10) | 5 (23) |
| Current IVDU -[% (n)] | 12 (23) | 8 (20) | 10 (43) |
| BMI (kg/m2) | 25.3 (21.4–28.9) | 25.6 (23.4–29.7) | 25.4 (22.7–29.3) |
| Waist circumference (cm) | 81 (75–91) | 87 (80–97) | 86 (78–95) |
| Diabetes [% (n)] | 6 (11) | 4 (11) | 5 (22) |
| Chronic renal disease [% (n)] | 14 (26) | 3 (8) | 8 (34) |
| AST (IU/l) | 53 (34–84) | 42 (33–60) | 46 (33–68) |
| ALT (IU/l) | 35 (23–63) | 37 (26–62) | 36 (25–62) |
| Follow-up time (years) | 4.3 (1.8–7.4) | 8.5 (5.7–10.7) | 6.6 (3.2–9.8) |
ALT, alanine aminotransferase; ART, antiretroviral therapy; AST, aspartate aminotransferase; HBsAg, surface antigen; HCV, hepatitis C virus; IVDU, intravenous drug use; WIHS, Women’s Interagency HIV Study.
Median (interquartile range) unless otherwise noted. Values are for index visit unless otherwise indicated.
The following characteristics had missing data: pre-HAART HIV peak viral load, n = 2; pre-HAART nadir CD4, n = 1; HIV viral load at index visit, 0 = 20; CD4 cell count at index visit, n = 5; BMI, n = 13; waist, n = 291; current alcohol use, n = 9; and current IVDU, n = 8.
Aspartate aminotransferase-to-platelet ratio index and FIB-4 and all-cause mortality
Table 2 shows the association of APRI and FIB-4 at the last available visit with all-cause mortality in HCV/HIV-co-infected women receiving HAART, using Cox proportional hazard regression models. Of the 450 HCV/HIV-co-infected women in Table 1, relevant covariates and adequate follow-up in the WIHS study beyond the date of their initial fibrosis marker measurements were available in 422, of whom 186 were known to have died. Analysis of all-cause mortality was performed in these 422 women. Data are expressed as hazard ratios and their associated 95% confidence intervals (95% CI). We first developed a ‘null’ model that included HIV-related covariates known to be related to all-cause mortality, including pre-HAART nadir and current CD4 cell counts, log10 pre-HAART peak and current HIV viral load and clinical AIDS pre-HAART. Among these, current CD4 cell counts less than 200 cells/µl and current HIV viral load were significantly associated with all-cause mortality. Addition of APRI or FIB-4 to the model demonstrated that these liver fibrosis markers were independent and significant predictors of all-cause mortality (Table 2). When modeled as a categorical variable, APRI marker levels more than 1.5 (severe fibrosis) were significantly associated with an increased risk of all-cause mortality (hazard ratio 2.78, 95%CI 1.87–4.12), compared with women with low APRI (<0.5, mild fibrosis) levels. Furthermore, women categorized with FIB-4 marker levels more than 3.25 (severe fibrosis) had significantly increased risk of all-cause mortality, compared with women with low FIB-4 (<1.5) levels (hazard ratio 2.58, 95% CI, 1.68–3.95). For the categorical expressions of APRI and FIB-4, only the highest of the three fibrosis levels (severe fibrosis) was significantly associated with increased all-cause mortality (Table 2).
Table 2.
Relative hazard ratios for all-cause mortality with 95% confidence interval conditional on survival to age 35 years among HIV/hepatitis C virus-co-infected Women’s Interagency HIV Study participants receiving HAART (n = 422 with 186 deaths).
| Null model | Adding APRI to the model |
Adding FIB-4 to the model |
|
|---|---|---|---|
| (a) Categorical variablea | |||
| Pre-HAART nadir CD4 cell counts (cells/µl) | |||
| <100 | 0.80 (0.46–1.40) | 0.90 (0.52–1.57) | 0.88 (0.50–1.54) |
| 100–200 | 1.11 (0.67–1.85) | 1.13 (0.68–1.88) | 1.11 (0.67–1.85) |
| 200–350 | 0.89 (0.55–1.45) | 0.93 (0.57–1.53) | 0.94 (0.57,1.53) |
| >350 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Pre-HAART peak HIV viral load, per log10 increase) | 1.15 (0.92–1.44) | 1.19 (0.94–1.49) | 1.14 (0.91,1.43) |
| Clinical AIDS prior to HAART | 1.22 (0.89–1.69) | 1.19 (0.86–1.64) | 1.24 (0.90,1.71) |
| ART use prior to HAART | 1.14 (0.79–1.66) | 1.10 (0.76–1.60) | 1.09 (0.75–1.58) |
| Current CD4 cell count (cells/µl) | |||
| <100 | 5.35 (3.30–8.66) | 4.25 (2.60–6.96) | 4.02 (2.44–6.63) |
| 100–200 | 1.79 (1.09–2.93) | 1.46 (0.89–2.40) | 1.39 (0.84–2.30) |
| 200–350 | 1.44 (0.92–2.26) | 1.38 (0.88–2.16) | 1.29 (0.82–2.03) |
| >350 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Current HIV viral load, per log10 increase | 1.22 (1.06–1.39) | 1.22 (1.07–1.39) | 1.20 (1.05–1.37) |
| Average alcohol use over previous 3 years (drinks/week) | |||
| ≥14 | 0.96 (0.49–1.85) | 0.76 (0.39–1.50) | 0.84 (0.43–1.64) |
| 3–13 | 0.83 (0.54–1.27) | 0.85 (0.56–1.30) | 0.85 (0.56–1.29) |
| 1–2 | 0.70 (0.49–0.99) | 0.68 (0.48–0.97) | 0.71 (0.50–1.00) |
| 0 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Diabetes mellitus | 0.97 (0.62–1.53) | 0.86 (0.55–1.36) | 0.90 (0.57–1.43) |
| Current IVDU | 1.37 (0.84–2.24) | 1.34 (0.81–2.20) | 1.28 (0.78–2.10) |
| Current (last available) APRI | |||
| Mild, <0.5 | 1.00 (reference) | ||
| Moderate, 0.5–1.5 | 1.01 (0.69–1.47) | ||
| Severe, >1.5 | 2.78 (1.87–4.12) | ||
| Current (last available) FIB-4 | |||
| Mild, <1.5 | 1.00 (reference) | ||
| Moderate, 1.5–3.25 | 1.09 (0.71–1.67) | ||
| Severe, >3.25 | 2.58 (1.68–3.95) | ||
| −2 LLa | 1576.86 | 1542.17 | 1546.57 |
| (b) Continuous variable | HR | ||
| Current (last available) APRI, per 0.25 increase from the index visit median of 0.59 | 1.19 (1.12–1.26) | ||
| Current (last available) FIB-4, per 0.5 increase from the index visit median of 1.66 | 1.22 (1.16–1.29) | ||
These estimates were very similar when APRI and FIB-4 were entered in the models as continuous variables and were not included in the table.
APRI, aspartate aminotransferase-to-platelet ratio index; ART, antiretroviral therapy; FIB-4,; IVDU, intravenous drug use.
Estimates of relative hazard ratios for other variables in the models reflect the values for APRI and FIB4 entered as categorical variables.
−2LL represents −2 log likelihood to assess how well the model fits the data.
When modeled as a continuous variable (Table 2b), an increase in the APRI marker level of 0.25 from the median index visit APRI level of 0.59 was associated with a hazard ratio of 1.19 (95% CI 1.12–1.26) for all-cause mortality. At the first (APRI = 0.39) and third (APRI = 1.07) quartile levels, increases in APRI of 0.25 were associated with hazard ratios of 1.27 (95% CI 1.17–1.39) and 1.11 (95% CI 1.07–1.15), respectively. Similarly, increases in FIB-4, expressed as a continuous variable, were also associated with a significantly increased risk of all-cause mortality. An increase in FIB-4 of 0.50 from the median index visit FIB-4 level of 1.66 was associated with a hazard ratio of 1.22 (95% CI 1.16–1.29). At the first (FIB-4 = 1.19) and third (FIB-4 = 2.64) quartile levels, increases in FIB-4 of 0.50 were associated with hazard ratios of 1.31 (95% CI 1.22–1.41) and 1.14 (95% CI 1.10–1.18), respectively.
The death rate per 1000 patient-years increased with increasing fibrosis marker severity. There were a total of 69 deaths in the severe APRI group and 89 deaths in the severe FIB-4 group. Specifically, for mild (<0.5), moderate (0.5–1.5) and severe (>1.5) fibrosis as measured by APRI, the death rates were 43.7, 54.9 and 171.2 per 1000 patient-years, respectively. Similarly, for mild (<1.5), moderate (1.5–3.25) and severe (>3.25) fibrosis as measured by FIB-4, the death rates were 34.8, 51.3 and 167.9 per 1000 patient-years, respectively. The highest death rates occurred among women with the highest APRI and FIB-4 marker levels.
Longitudinal patterns of liver-related and nonliver-related mortality and serum fibrosis marker levels
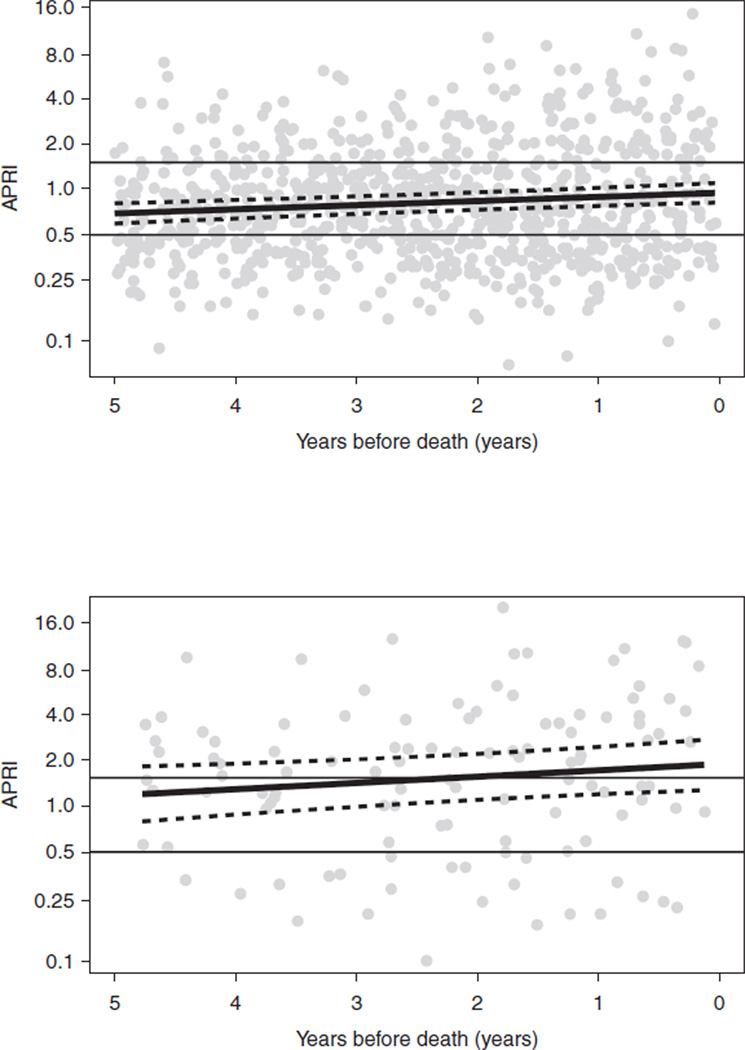
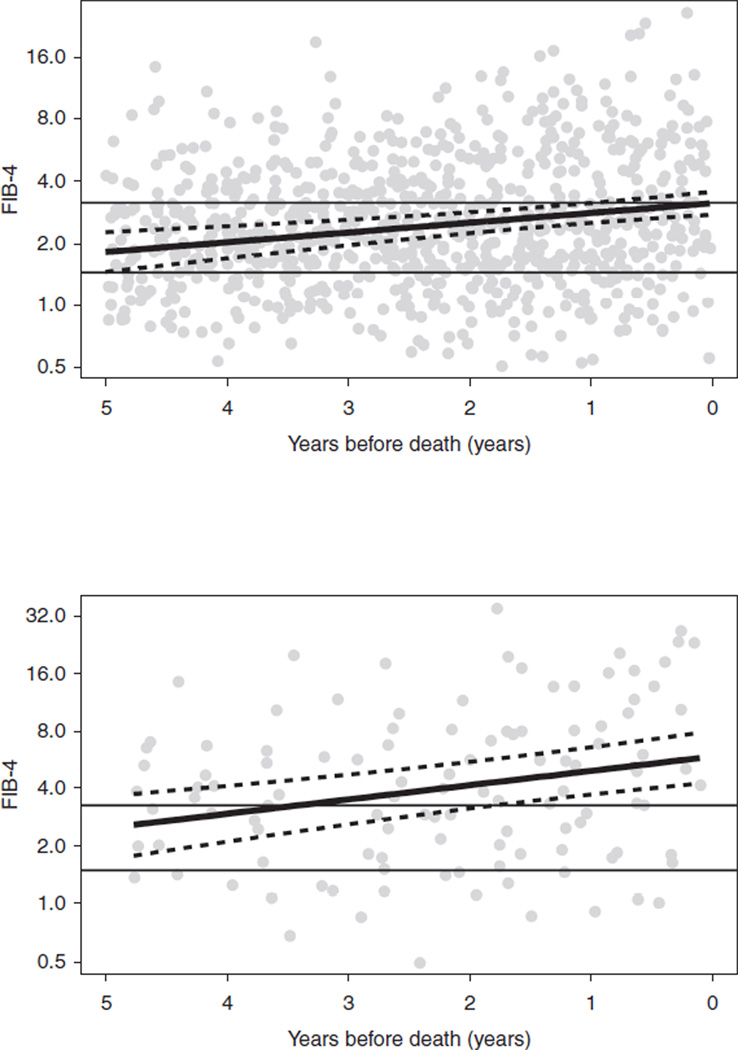
Of the 450 HCV/HIV-co-infected women, there were 153 women who died and had at least two fibrosis marker assessments (APRI or FIB-4) available within 5 years of their death. Deaths for 133 of these women were classified as nonliver-related (as determined by the ‘primary cause of death’ on the death certificates) and 20 as liver-related. The median age (IQR) of those who died of liver-related and nonliver-related deaths was 49 (43–54) and 50 (46–54) years, respectively. An initial model including age at death as a predictor, indicated age was not significant for either APRI (P = 0.07) or FIB-4 (P = 0.29), so this effect was removed from the final model. For both APRI and FIB-4, the difference between the smooth trends for liver-related versus nonliver-related deaths was highly significant (P < 0.001 for both markers). For each decedent, APRI and FIB-4 marker levels measured within 5-years of their death are presented in Figs 1 and 2, respectively. Within each figure, the top panel includes women who died of nonliver-related deaths (n = 133) and the bottom panel includes women who died of liver-related deaths (n = 20).
Fig. 1. Aspartate aminotransferase-to-platelet ratio index data for 153 hepatitis C virus/HIV-co-infected women on HAART who died and had at least two fibrosis marker assessments (aspartate aminotransferase-to-platelet ratio index or FIB-4) available within 5 years of their death.
The figure depicts aspartate aminotransferase-to-platelet ratio index (APRI) data from the last available fibrosis marker assessment up to 5 years before death for 133 nonliver-related (a: upper panel) and for 20 liver-related (b: lower panel) deaths. The solid, black curve is the regression line. APRI is depicted on a log-scale with cut-off lines for low (<0.5) and high (>1.5) values.
Fig. 2. FIB-4 data for 153 hepatitis C virus/HIV-co-infected women on HAART who died and had at least two fibrosis marker assessments (aspartate aminotransferase-to-platelet ratio index or FIB-4) available within 5 years of their death.
The figure depicts FIB-4 data from the last available fibrosis marker assessment up to 5 years before death for 133 nonliver-related (a: upper panel) and for 20 liver-related (b: lower panel) deaths. The solid, black curve is the regression line. FIB-4 is depicted on a log-scale with cut-off lines for low (<1.5) and high (>3.25) values.
The longitudinal analysis demonstrated that both APRI and FIB-4 fibrosis marker measurements increased during the 5 years prior to death for all women; however, the slope of the increase was greater for women who died a liver-related death compared with nonliver-related death. For APRI, the estimated slopes (adjusting for nonlinear curvature) were −0.0142 (P = 0.94) for nonliver-related death and 1.201 (P = 0.025) for liver-related death. For FIB-4, the estimated slopes were 0.042 (P = 0.78) for nonliver-related death and 0.837 for liver-related death (P = 0.054). In both the APRI and FIB-4 models, the nonlinear trends were significantly different between the two death groups (both P = 0.024). In addition, APRI and FIB-4 fibrosis marker levels were higher at all time points during the 5 years prior to death in women who died a liver-related death (Figs 1 and 2 lower panel), compared with women whose cause of death was nonliver-related (Figs 1 and 2 upper panel) (P = 0.001 for APRI and FIB-4).
Discussion
This study showed that FIB-4 and APRI were strongly associated with all-cause mortality in HCV/HIV-co-infected women. Both fibrosis markers utilize standard laboratory values that are simple and regularly performed in clinical care of patients with HIV or HCV and include AST, ALT and platelets. Assessment of the degree of hepatic fibrosis carries important prognostic information and has therapeutic implications with respect to timing of HCV treatment and monitoring for evidence of complications of portal hypertension. If women have no liver fibrosis, they may elect to await newer all oral HCV therapies, but if they have severe fibrosis (fibrosis stage 3–4) they should undergo current treatment for HCV.
Although liver biopsy is considered by clinicians to be the most useful method to determine the amount of hepatic fibrosis and inflammation, it is an imperfect test, providing a small piece of liver with nonuniform histologic findings. Smaller tissue samples generally underestimate fibrosis [14–17]. In WIHS, only 20% of HCV/HIV-co-infected women have undergone liver biopsy and fewer (1.8%) have received therapy for HCV. WIHS is not a treatment study, but an observational cohort. However, women are encouraged to seek treatment for HCV by WIHS investigators and we have provided educational programs, so that participants can learn about their HCV, liver disease and possible therapies.
In light of these limitations of liver biopsy, there has been interest and progress made in the development of noninvasive markers of hepatic fibrosis. One of these indices, FIB-4, was developed and validated in a cohort of patients co-infected with HCV/HIV [9]. This study found that FIB-4 had an accuracy of 0.77 for differentiating between Ishak fibrosis stage 0–3 and Ishak fibrosis stage 4–6 among patients co-infected with HCV/HIV [18]. APRI has been demonstrated to predict significant fibrosis and cirrhosis, with accuracies of 0.80 and 0.89, respectively, in patients with HCV infection alone [8] and somewhat decreased accuracy (0.71–0.73 for cirrhosis) in the co-infected populations [19–21]. The serological noninvasive methods of fibrosis assessment can be performed on small volume blood samples and can be automated and repeated frequently and retrospectively. Other markers of mortality risk include the model for end-stage liver disease [22] and Child–Pugh–Turcotte scores [23]. In patients with known cirrhosis (F4) and decompensated liver disease, they have been used to assess mortality risk for surgery and need for liver transplantation. They are not used in patients without cirrhosis in contrast to APRI and FIB-4, which assess the amount of liver fibrosis from none (F0) to cirrhosis (F4).
Death certificates have certain limitations and those ‘nonliver deaths’ in HCV/HIV-co-infected women may have had significant contribution from liver disease: for example, the contribution of hepatic disease may not have been noted in septic deaths, renal deaths and multisystem organ failure [12]. In addition, not all abnormalities leading to changes in ALT, AST and platelets can be assumed to be due to hepatic fibrosis progression per se, as hematologic disorders and medication effects, likely more common in HIV, may cause changes. As noted above, WIHS is an observational study without patient visits, so we do not have data for APRI or FIB-4 in hospitalized women or women who missed visits. Of interest, there were higher rates of death in women with severe fibrosis scores, suggesting that liver fibrosis played a role even in women deemed to have died from HIV. Indeed, our results demonstrate a significant association between increased levels of APRI and FIB-4 and the risk of death from any cause. Other studies have shown that HCV/HIV co-infection is associated with higher mortality than HIV alone [5]. APRI and FIB-4 do not distinguish the cause of fibrosis. Patients with HCV have increased hepatotoxicity from HAART [24], and those with cirrhosis have altered metabolism of drugs.
Our study is the first longitudinal study of noninvasive markers of fibrosis solely in women co-infected with HCV and HIV. The fibrosis markers were predictive of all-cause mortality, but were abnormal some years before death and were higher in women dying a liver-related death. This suggests that these noninvasive markers can be of practical value in all women co-infected with HIV and HCV to determine whether liver disease is progressing and, if so, to reinforce the need for therapy for HCV. These noninvasive markers have been shown to predict liver-related death in a smaller cohort of HIV-infected individuals, predominantly men [25]. During the 5 years prior to death, our data showed higher overall fibrosis marker (APRI and FIB-4) levels, as well as more rapid increases in fibrosis marker levels in women who experienced a liver-related death compared with women who experienced a nonliver-related death. As the components of these indirect fibrosis tests are readily available as part of clinical care, they can be used to assess progression of liver fibrosis and even risk of mortality in co-infected women. This may lead patients and their providers to more strongly consider HCV therapy. Better, noninvasive methods for assessment of liver fibrosis are needed to provide timely selection of women whose liver disease is progressing and who are, therefore, in more urgent need of therapy for HCV.
Acknowledgements
The number of authors on this manuscript reflects the six sites who collected the data and the data analysis center in this multicenter cohort study. All authors were involved in collection of data, data analysis and writing and review of the manuscript. K.B. and M.G.P. were the primary leaders on this manuscript, being involved in generation of the hypotheses and every aspect of data analysis, writing and review of the manuscript. C.P., C.C. and S.J.G. were involved in data cleaning, data analysis and writing and review of the manuscript. A.L.F., P.C.T., M.A., M.J.G., M.C.V. and M.P. were involved in collection of data, review of the data analysis plan and review of the manuscript. G.B.S. and H.D.S. were involved in review of the data analysis plan and review of the manuscript.
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, New York (Howard Minkoff); Washington, District of Columbia Metropolitan Consortium (Mary Young); the Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); and Data Coordinating Center (S.J.G.). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse and the National Institute on Deafness and Other Communication Disorders.
M.P. is supported by NIAID R21 A1088361. P.C.T. is supported by NIAID K23 66943 and this work is supported with resources and the use of facilities at the San Francisco CA Veterans Affairs Medical Center.
Footnotes
Conflicts of interest
There are no conflicts of interest involved in this manuscript.
References
- 1.Koziel MJ, Peters MG. Viral hepatitis in HIV infection. N Engl J Med. 2007;356:1445–1454. doi: 10.1056/NEJMra065142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 3.Benhamou Y, Bochet M, Di MV, Charlotte F, Azria F, Coutellier A, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 4.Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. Irish Hepatology Research Group. N Engl J Med. 1999;340:1228–1233. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]
- 5.Weber R, Sabin CA, Friis-Moller N, Reiss P, El Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 6.Sulkowski MS, Mehta SH, Torbenson MS, Higgins Y, Brinkley SC, de Oca RM, et al. Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. AIDS. 2007;21:2209–2216. doi: 10.1097/QAD.0b013e3282f10de9. [DOI] [PubMed] [Google Scholar]
- 7.Al Mohri H, Murphy T, Lu Y, Lalonde RG, Klein MB. Evaluating liver fibrosis progression and the impact of antiretroviral therapy in HIV and hepatitis C coinfection using a noninvasive marker. J Acquir Immune Defic Syndr. 2007;44:463–469. doi: 10.1097/QAI.0b013e318030ff8e. [DOI] [PubMed] [Google Scholar]
- 8.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 9.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 10.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 11.Hessol NA, Schneider M, Greenblatt RM, Bacon M, Barranday Y, Holman S, et al. Retention of women enrolled in a prospective study of human immunodeficiency virus infection: impact of race, unstable housing, and use of human immunodeficiency virus therapy. Am J Epidemiol. 2001;154:563–573. doi: 10.1093/aje/154.6.563. [DOI] [PubMed] [Google Scholar]
- 12.French AL, Gawel SH, Hershow R, Benning L, Hessol NA, Levine AM, et al. Trends in mortality and causes of death among women with HIV in the US: A ten-year study. J Acquir Immune Defic Syndr. 2009;51:399–406. doi: 10.1097/QAI.0b013e3181acb4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruppert D, Wand MP, Carroll RJ. Semiparametric regression. Cambridge Series in Statistical and Probabilistic Mathematics. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- 14.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Rousselet MC, Michalak S, Dupre F, Croue A, Bedossa P, Saint-Andre JP, et al. Sources of variability in histological scoring of chronic viral hepatitis. Hepatology. 2005;41:257–264. doi: 10.1002/hep.20535. [DOI] [PubMed] [Google Scholar]
- 16.Skripenova S, Trainer TD, Krawitt EL, Blaszyk H. Variability of grade and stage in simultaneous paired liver biopsies in patients with hepatitis C. J Clin Pathol. 2007;60:321–324. doi: 10.1136/jcp.2005.036020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 18.Ishak KG. Chronic hepatitis: morphology and nomenclature. Mod Pathol. 1994;7:690–713. [PubMed] [Google Scholar]
- 19.Macias J, Giron-Gonzalez JA, Gonzalez-Serrano M, Merino D, Cano P, Mira JA, et al. Prediction of liver fibrosis in human immunodeficiency virus/hepatitis C virus coinfected patients by simple noninvasive indexes. Gut. 2006;55:409–414. doi: 10.1136/gut.2005.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelleher TB, Mehta SH, Bhaskar R, Sulkowski M, Astemborski J, Thomas DL, et al. Prediction of hepatic fibrosis in HIV/HCV coinfected patients using serum fibrosis markers: the SHASTA index. J Hepatol. 2005;43:78–84. doi: 10.1016/j.jhep.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Tural C, Tor J, Sanvisens A, Perez-Alvarez N, Martinez E, Ojanguren I, et al. Accuracy of simple biochemical tests in identifying liver fibrosis in patients co-infected with human immunodeficiency virus and hepatitis C virus. Clin Gastroenterol Hepatol. 2009;7:339–345. doi: 10.1016/j.cgh.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 23.Pugh RN. Pugh’s grading in the classification of liver decompensation. Gut. 1992;33:1583. doi: 10.1136/gut.33.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.den Brinker M, Wit FW, Wertheim-van Dillen PM, Jurriaans S, Weel J, van Leeuwen R, et al. Hepatitis B and C virus co-infection and the risk for hepatotoxicity of highly active antiretroviral therapy in HIV-1 infection. AIDS. 2000;14:2895–2902. doi: 10.1097/00002030-200012220-00011. [DOI] [PubMed] [Google Scholar]
- 25.Nunes D, Fleming C, Offner G, Craven D, Fix O, Heeren T, et al. Noninvasive markers of liver fibrosis are highly predictive of liver-related death in a cohort of HCV-infected individuals with and without HIV infection. Am J Gastroenterol. 2010;105:1346–1353. doi: 10.1038/ajg.2009.746. [DOI] [PubMed] [Google Scholar]