Abstract
Background
Rice is the world's most important cereal crop and phosphorus (P) and zinc (Zn) deficiency are major constraints to its production. Where fertilizer is applied to overcome these nutritional constraints it comes at substantial cost to farmers and the efficiency of fertilizer use is low. Breeding crops that are efficient at acquiring P and Zn from native soil reserves or fertilizer sources has been advocated as a cost-effective solution, but would benefit from knowledge of genes and mechanisms that confer enhanced uptake of these nutrients by roots.
Scope
This review discusses root traits that have been linked to P and Zn uptake in rice, including traits that increase mobilization of P/Zn from soils, increase the volume of soil explored by roots or root surface area to recapture solubilized nutrients, enhance the rate of P/Zn uptake across the root membrane, and whole-plant traits that affect root growth and nutrient capture. In particular, this review focuses on the potential for these traits to be exploited through breeding programmes to produce nutrient-efficient crop cultivars.
Conclusions
Few root traits have so far been used successfully in plant breeding for enhanced P and Zn uptake in rice or any other crop. Insufficient genotypic variation for traits or the failure to enhance nutrient uptake under realistic field conditions are likely reasons for the limited success. More emphasis is needed on field studies in mapping populations or association panels to identify those traits and underlying genes that are able to enhance nutrient acquisition beyond the level already present in most cultivars.
Keywords: Adventitious roots, deoxymugineic acid, marker-assisted selection, nutrient-use efficiency, phosphorus transporters, phosphorus uptake, zinc uptake, low-molecular-weight organic acids, radial oxygen loss, radical oxygen stress, root hairs, quantitative trait loci, QTL
INTRODUCTION
Phosphorus (P) deficiency and zinc (Zn) deficiency are two of the most important nutritional constraints to rice growth across the globe (Ismail et al., 2007). The desire to improve the Zn and P acquisition efficiency of rice roots arises because P and Zn fertilizers are not always adequate to overcome the crop production constraints. Fertilizers are a costly input, such that their use limits the profitability of rice farming for high-input or low-input systems, and the use of fertilizers for these two rice nutrients is notoriously inefficient. Both fertilizer-P and fertilizer-Zn can rapidly form insoluble complexes in the soil, rendering them unavailable for plant uptake unless genotypes with an improved capability to access these soil-bound nutrients are developed (Fageria et al., 1988; Johnson-Beebout et al., 2009). While the size of remaining global reserves of rock P is often contested and possibly not as dire as earlier reports suggested (e.g. Steen, 1998), P still remains a finite resource and most of the rock P reserves are controlled by relatively few countries (Cordell et al., 2009). Further, the inefficient use of P fertilizer leads to pollution because erosion of soils high in P strongly contributes to eutrophication of waterways (Sharpley et al., 2001). Breeding crop cultivars with enhanced capacity to acquire Zn and P from native and fertilizer sources has therefore been advocated as a cost-effective means by which to improve the efficiency of nutrient use in rice production (Ismail et al., 2007).
Rice-growing environments can be categorized into two very broad categories: ‘upland’ and ‘lowland’. For the purposes of this review, ‘lowland’ rice production is characterized by soil submergence during crop establishment and/or the vegetative and early reproductive growth stages. ‘Upland’ rice production is characterized by soil that may be saturated, but is not intentionally submerged during any part of the growing season. Phosphorus deficiency is more common in upland environments, because P predominantly reacts with iron (Fe) and aluminum (Al) in acid soils, and calcium (Ca) in neutral to alkaline soils to form poorly soluble complexes (Holford, 1997), but is released from some of these compounds after flooding as the redox potential and pH change. After long-term flooding or seasonal drainage of a rice paddy, some of this plant-available P becomes unavailable again as it binds to or precipitates with newly forming Fe oxide compounds (Kirk, 2004). In contrast, Zn deficiency is more common in lowland environments because the low redox potential causes Zn to precipitate as zinc sulphide, zinc carbonate or zinc oxy-hydroxides, whereas in aerobic soils Zn2+ is readily available to plants often on cation exchange sites. One exception is that calcareous soils tend to be Zn-deficient in aerobic conditions as well as long-term anaerobic conditions, with Zn most readily available after short-term flooding at an intermediate redox potential. Interested readers are referred to earlier reviews of the details of Zn and P chemistry in soils (Kirk, 2004; Impa and Johnson-Beebout, 2012).
While plant requirements for P and Zn vary by orders of magnitude and their roles within plants are contrasting, root traits that enhance their acquisition are similar. Because both nutrients have poor mobility in soils, many traits that enhance the capture of P will also improve Zn acquisition, although one trait may be more effective in upland environments (and upland genotypes) for the purpose of P acquisition, while being more effective in lowland environments for Zn acquisition. These root traits are the subject of this review, and discussion focuses on the prospects of these traits to be exploited in plant breeding using a marker-assisted selection (MAS) approach.
Although it is often emphasized that enhancing nutrient-use efficiency is critical across the globe both to sustain yields in low-input agriculture and to reduce fertilizer inputs in high-input agriculture, it is not clear if traits that enhance efficiency in low-input systems also confer efficiency in high-input systems. Most of our understanding of root traits that enhance the acquisition of P and/or Zn arises from studies that have examined plants suffering a relatively high level of stress, rather than testing differences in plant uptake efficiency under nutrient-sufficient conditions. There is little or no evidence to suggest that genes or traits that confer greater acquisition of P/Zn (and therefore greater crop growth) in P or Zn-deficient soils (i.e. 1–3 t ha−1 rice crops in Asia or India) will also help high-input farmers maintain 8-t yields with reduced fertilizer inputs. While there are several examples of the introduction in breeding programmes of root traits that enhance nutrient uptake from nutrient-impoverished soils (see Wissuwa et al., 2009), we are not aware of any examples of breeding for root traits to enhance nutrient uptake and thereby reduce fertilizer requirements of high-yielding crops. Thus, this review focuses on the potential of a range of plant traits to be exploited in breeding programmes for low-input systems, and their applicability to enhancing fertilizer-use efficiency remains to be tested.
ROOT TRAITS TO INCREASE THE POOL OF MOBILE P AND Zn
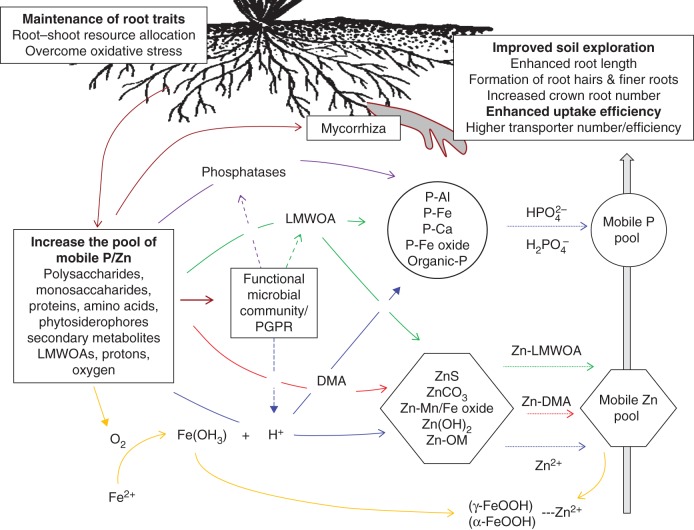
Given that a large proportion of P and Zn in many upland- and lowland-rice soils exist as insoluble complexes, root traits that confer the capacity to mobilize a proportion of P and/or Zn are highly desirable. Traits that can potentially enhance the mobilization of P and Zn from soils have received significant attention across a range of crop species [see reviews by Hinsinger (2001), Lambers et al. (2006) and Richardson et al. (2011) for P, and Broadley et al. (2007) for Zn]. The major traits that have been pursued for both Zn and P solubilization include root efflux of low-molecular-weight organic acids (LMWOAs) and efflux of H+ ions, as well as efflux of other chelating agents (e.g. siderophores for Zn) or phosphatases capable of mobilizing organic P in soils (Fig. 1).
Fig. 1.
Possible root induced mechanisms for P and Zn uptake from rhizosphere in rice. Abbreviations: LMWOA, low-molecular-weight organic acid; DMA, deoxy mugineic acid; OM, organic matter; α-FeOOH and γ-FeOOH, iron oxyfied lepidocrocite and goethite on roots (root plaques); P, phosphate. Dashed lines with arrow indicate P/Zn uptake by roots.
Efflux of ligands from roots
Among the most widely studied ligands exuded from plant roots are the LMWOAs, which compete with inorganic P (Pi) for the same sorption sites (ligand exchange) or solubilize Pi via ligand-promoted mineral dissolution in acid soils dominated by Al and Fe (Schefe et al., 2008). Protons released to balance the negative charge of LMWOAs may further promote Pi release from soil minerals (see following section).
LMWOAs have been implicated in both P and Zn acquisition in upland and lowland rice. Modelling studies suggest that root efflux of citrate plays a key role in mobilization of P in aerobic rice soils (Kirk, 1999; Kirk et al., 1999a, b). The model used by Kirk (1999) is based on observations that rice plants were able to obtain P from the alkali-soluble inorganic soil P (NaOH Pi) fraction (Hedley et al., 1994), and this mobilization of P could not be explained by either rhizosphere pH changes or by the release of phosphatases from roots. In addition, physiological studies showed that P-deficient rice plants increased the efflux of citrate compared with P-replete plants (Kirk et al., 1999a). However, while the modelling studies show good agreement between predicted P uptake and measured P uptake in soil-grown plants (Kirk et al., 1999b), it is worth noting that no direct cause and effect relationship between LMWOA efflux and P uptake has been shown for upland rice. In this regard rice is similar to other upland crops, where evidence for the benefits of LMWOA efflux is often circumstantial. For example, wheat can readily deplete the NaOH Pi fraction (Vu et al., 2008; Rose et al., 2010) but it is possible that the NaOH Pi pool is simply in rapid equilibrium with the labile bicarbonate Pi fraction such that no active P solubilization is necessary.
Genotypic variation for root-efflux of LMWOAs under P deficiency has also been demonstrated in rice (Kirk et al., 1999; Hoffland et al., 2006); however, we are unaware of any studies that have directly linked this efflux to genotypic P efficiency in the field, and published studies have been inconclusive. Hoffland et al. (2006) found higher citrate exudation in genotypes that grew relatively well with rock phosphate as a P source, yet Kirk et al. (1999a, b) reported lowest citrate exudation in the P-efficient Brazilian upland rice variety ‘IAC47’ and the data of Kirk et al. (1999a, b) also suggested that citrate exudation did not necessarily increase under P deficiency. To this end, the results from studies on rice mirror those in other cereals: a recent review by Richardson et al. (2011) concluded that despite the hopes held for LMWOAs in improving P nutrition of crops, little evidence exists in species that do not form cluster roots. Further, recent studies have questioned whether the low concentrations of LMWOAs effluxed from cereal roots can effectively compete with P for the same sorption sites, particularly in the presence of numerous unsaturated anionic binding domains found in Al- and Fe-(oxy)hydroxide-dominated acid soils (Schefe et al., 2008; Oburger et al., 2009). This LMWOA competition for P sorption sites is more effective at releasing P when there is a relatively high amount of P present (Johnson and Loeppert, 2006), implying that this root trait may be more likely to increase P fertilizer-use efficiency than P uptake from extremely deficient soils.
Studies on LMWOA efflux from rice roots in response to Zn deficiency are equally inconclusive. While higher root-efflux rates of citrate (Hajiboland et al., 2005; Hoffland et al., 2006) and malate (Hajiboland et al., 2005; Gao et al., 2009; Rose et al., 2011a) have been observed in Zn-efficient upland and lowland-rice genotypes suffering from Zn deficiency, two issues need to be resolved before one could conclude that this efflux directly enhances Zn uptake. First, the efflux of LMWOAs often coincides with radical oxygen stress-induced root leakage and may therefore be a consequence of root membrane damage rather than an active stress response (Chen et al., 2009; Rose et al., 2011a, 2012). The second unresolved question relates to the amount of LMWOAs needed in the rhizosphere to release significant amounts of Zn. There is very little information available regarding how the observed root exudate efflux rates in nutrient solution correspond to their concentration in rhizosphere soil. From the few available direct measurements of rhizosphere LMWOA concentrations, we know that malate may be found in concentrations in the order of magnitude 0·1–1 mm, with a wider range measurable in a citrate experiment indicating 0·01–1 mm (Kirk et al., 1999a; Gao et al., 2009). This information suggests a very wide range of rhizosphere root exudate concentrations among different types of exudates. While two soil incubation experiments have shown that the concentrations of citrate and malate released from the roots of Zn-deficient rice plants were insufficient to mobilize significant amounts of Zn from two Zn-deficient soils (Gao et al., 2009; Rose et al., 2011a), the effectiveness of a specific exudate is likely to vary in different soil types (Impa and Johnson-Beebout, 2012), so further research is needed to determine which root exudates are potentially the most useful trait.
Taken as a whole, it can be concluded that rice plants tend to increase exudation of LMWOAs, particularly citrate and malate, under P- or Zn-deficiency stress. What is unclear, however, is whether the increased efflux of citrate or malate provides direct benefits in terms of increased P or Zn acquisition from soils. It is also unclear whether the enhanced LMWOA efflux is a strategy employed specifically by P- or Zn-efficient genotypes and could therefore be exploited to breed more efficient crop cultivars, or whether it is simply a general stress response that does not necessarily confer tolerance to P- or Zn-deficiency stress.
One ligand that does show promise as a breeding target for improving Zn acquisition by rice is the phytosiderophore deoxymugineic acid (DMA) which is released by graminaceous plants, particularly in response to Fe deficiency. Overexpressing the DMA efflux transporter OsTOM1 has been shown to enhance tolerance to Fe deficiency under controlled conditions (Nozoye et al., 2011). Other studies showed that these effects would also extend to Zn uptake (Ishimaru et al., 2011) and some indirect lines of evidence suggest that higher tolerance to Zn deficiency of some genotypes was due to enhanced DMA exudation and subsequent uptake of the Zn–DMA complex (Arnold et al., 2010; Widodo et al., 2010; Ptashnyk et al., 2011). It is important to note that efflux of DMA from rice roots appears to decline as Zn-deficiency stress increases (Suzuki et al., 2008); however, we have recently discovered that Zn-efficient genotypes tend to maintain DMA efflux at higher rates for a longer period after Zn-deficiency-stress conditions are imposed (M. T. Rose, unpubl. data). In this way it is possible that higher DMA efflux rates are actually a consequence of superior internal Zn efficiency or ability to avoid a ‘panic’ response under Zn deficiency (see ‘General response to stress – stress response versus tolerant response’ below). Although no direct measurements of DMA concentration in rhizosphere soil have yet been published, it has been modelled from the efflux rate and root architecture, ranging from 0·001 to 0·01 mm, which is several orders of magnitude lower than the measured LMWOA concentrations mentioned above (Ptashnyk et al., 2011). As with the LMWOAs, it is unclear how effective this low concentration might be at mobilizing Zn from soil, and its effectiveness is likely to differ with changing soil chemistry.
It is interesting to note that enhanced phytosiderophore release from roots has not been exploited in upland crops, and there are conflicting reports regarding their role in genotypic Zn acquisition efficiency in aerobic soils (reviewed by Hacisalihoglu and Kochian, 2003). Indeed, these authors concluded that differences in internal use of Zn were the most likely causes of genotypic differences in Zn efficiency rather than differences in Zn acquisition from soils (Hacisalihoglu and Kochian, 2003). Given that Zn acquisition appears to drive Zn efficiency in lowland rice (Wissuwa et al., 2006), it is important to find out if exudation of LMWOAs or DMA is actually triggered as a low-Zn stress response.
Rhizosphere acidification
Acidification of the rhizosphere has been shown to be effective in mobilizing P in a wide range of flooded soils (Kirk and Saleque, 1995). Two main processes are involved in rhizosphere acidification: (1) the radial oxygen loss (ROL) from roots oxidizes Fe2+ to release two protons [4Fe2+ + O2 +10H2O → 4Fe(OH)3 + 8H+]; and (2) the imbalance of cation/anion uptake due mainly to the presence of N as NH4+ in reduced soil (Begg et al., 1994), which results in greater release of H+ ions into the rhizosphere. The possibility also exists that H+ efflux is an active mechanism in its own right, and efflux of anions or ligands (LMWOAs) may act to balance the increased H+ efflux. Regardless, any of these mechanisms would release P or Zn in neutral to alkaline lowland soils; however, to what extent they could be exploited in breeding is unclear. We are not aware of any evidence suggesting certain rice genotypes would increase cation/anion imbalances above the level normally observed in flooded soils, particularly since nitrogen (N) dynamics are primarily controlled by soil factors.
Compared with direct proton efflux from roots, the P-mobilizing effect of rhizosphere acidification by ROL in flooded soils is more complex. ROL from roots and the subsequent oxidation of Fe2+ to Fe-(hydr)oxides leads to two competing processes: (1) the mobilization of P from acid soluble P pools (presumably Ca-P minerals) as H+ is released from the oxidation of Fe2+; and (2) the immobilization of P as it is absorbed on Fe-(hydr)oxides that form at the root surface. The modelling study of Kirk and Saleque (1995) presumed that there were net benefits in terms of P mobilization, and the agreement between model predictions and measured P uptake seemingly confirmed this assumption. Further, aerenchyma formation (i.e. root porosity) in rice plants increases under P deficiency (Kirk and Le Van Du, 1997) and, given that root porosity is directly proportional to ROL from roots (Mei et al., 2009), it is possible that increased ROL is a plant adaptation to P stress. However, the formation of aerenchyma is also a strategy used by plants to increase root exploration of the soil at a lower carbon cost (Lynch, 2007), and the increased root porosity under P deficiency stress reported by Kirk and Le Van Du (1997) coincided with an increase in the proportion of fine roots. It is therefore possible that increased ROL is a consequence of plants producing a low-carbon-cost root system under P deficiency, rather than a direct strategy to acidify the rhizosphere and solubilize P. This may be consistent with the fact that genotypic differences in ROL from roots have been reported (Mei et al., 2009) but it is not known whether genotypes that had higher ROL from roots showed greater acquisition of P.
There is further uncertainty about the role of the Fe plaque formed by the ROL from roots in the acquisition of nutrients. For example, some studies suggest that the Fe plaque acts as a reservoir for P and Zn (Zhang et al., 1998, 1999), while other studies suggest it may act as a barrier to nutrient uptake (discussed in Liang et al., 2006) or suggest that the Fe plaque has little relevance to nutrient acquisition because it is not present on the fine young roots involved in nutrient uptake (Seyfferth et al., 2010). That Fe plaque was not observed on the fine young roots involved in nutrient uptake is interesting in itself, given that these fine roots have higher rates of ROL than primary roots which typically form non-permeable barrier to ROL (Kirk, 2003). Until we gain a deeper understanding of the role of ROL and the Fe plaque in nutrient uptake, and whether genotypic differences in either ROL or Fe plaque formation confer enhanced P or Zn acquisition under field conditions, there is little scope for selecting variants of these traits to improve nutrient acquisition in rice.
Efflux of phosphatases
Between 30 and 70 % of P in soils can be in organic (Po) forms (Harrison, 1987) including inositol phosphates, phospholipids, sugar phosphates and nucleic acids. The desire to breed or engineer plants that possess an enhanced capacity to access these typically recalcitrant forms of P in soils has driven research into root-secreted phosphatase enzymes, but with limited breeding advances to date. Attempts to increase the efflux of various phosphatases through transgenic approaches have typically been unsuccessful, despite large increases in the amount of P-solubilizing enzyme secreted by roots (reviewed by Richardson et al., 2009). Indeed, there is still debate as to whether phosphatase activity in the rhizosphere or the physical availability of phytate in soils limits the bioavailability of soil Po to plant roots.
In rice plants numerous phosphatase genes are up-regulated in roots under P deficiency (Pariasca-Tanaka et al., 2009; Li et al., 2010); however, there is no evidence that the expression of these genes is higher in P-efficient genotypes (Pariasca-Tanaka et al., 2009). Further, phosphatases play a number of roles in planta and we are unaware of any evidence that the reported up-regulation of phosphatase genes in rice roots (Pariasca-Tanaka et al., 2009; Li et al., 2010) leads to higher efflux from roots into the soil. Until there is a greater understanding of root-secreted phosphatase–Po interactions in the soil the chances of exploiting root-phosphatase activity for greater nutrient acquisition appear slim.
Enrichment of P/Zn-mobilizing microorganisms
Microbial activity in the rice rhizosphere may either reduce the pool of mobile P in soil via the consumption of plant-derived carbon and plant/soil-derived soluble-P (immobilization), or augment the soluble-P pool through production of microbial ligands or phosphatases (Kirk et al., 1999b; Richardson and Simpson, 2011). Unfortunately, as far as we are aware, direct evidence for dominance of either mechanism is currently lacking. Moreover, it is highly likely that relevant microbial processes will be as spatially and temporally variable as is microbial community structure on the micro-scale (Ikenaga et al., 2003), and microbial processes will differ between soil types and plant genotypes on the macro-scale (Berg and Smalla, 2009). Systematic assessment may therefore be difficult. Nevertheless, indirect evidence for the role of microbes in rice nutrition is rapidly accumulating via the use of molecular biology techniques to describe and interpret rhizosphere microbial community dynamics (Sørensen et al., 2009), together with culture-dependent studies of plant-growth promoting microorganisms (Vessey, 2003).
The plasticity of microbial communities in response to rice plant nutritional status and even rice genotype is well documented (Hardoim et al., 2011). However, most of the work to date has focused on microbial communities involved in carbon and N cycling, rather than P or Zn. However, studies on N have confirmed that selective breeding for specific functional microbial communities is possible (Tan et al., 2003; Knauth et al., 2005), and Hardoim et al. (2011) recently showed that ‘traditional’ and ‘improved’ rice cultivars form distinct bacterial associations, supporting the view that plant breeding strategies targeting high-yield crops exert an effect on the root-associated bacterial communities.
It remains unknown as to the main mechanism/s by which rice genotypes select for specific communities over others; however, variations in root exudates, proton and oxygen efflux are hypothesized to play key roles (Ikenaga et al., 2003; Bais et al., 2006; Hartmann et al., 2009). Briones et al. (2002) compared the diversity and activities of ammonium-oxidizing bacteria in the rice root environment and found marked inter-varietal differences, which could be partly explained by differences of the oxygen concentrations around roots. With respect to P, de Oliveira et al. (2009a) recently found that the genetic and metabolic structure of the rhizosphere bacterial community of maize was strongly influenced by the level of P in the soil, and also to a smaller extent by the maize genotype. Interestingly, the bacterial communities from the rhizosphere of P-efficient maize genotypes under P stress could be discriminated from communities from non-efficient genotypes when grouped by their carbon nutrition profiles. In comparison, under high-P conditions, microbial communities were more variable and no trends could be discerned with respect to plant genotypic P-efficiency. In a related study, de Oliveira et al. (2009b) specifically investigated mycorrhizal communities in the roots of the same maize genotypes under low-P and high-P conditions. Similar results were obtained to the aforementioned study, with the P-efficient genotypes found to host an exclusive, consistent subset of mycorrhizal DNA, but only under P deficiency. In light of the fact that mycorrhiza are known to be actively involved in P acquisition in cereals, the results of de Oliveira et al. (2009b) lends support to the hypothesis for plant genotype-driven selection of functional microbial communities. In work comparing Zn-efficient and -inefficient wheat genotypes, Rengel (1997) found that Zn deficiency increased the numbers of fluorescent pseudomonads in the rhizosphere of all wheat genotypes tested, but the effect was particularly obvious for genotypes tolerant of Zn deficiency. These reports imply a significant relationship between nutrient-efficient genotypes, root exudation and microbial community selection, but raise a question as to whether the observed microbial communities actively contribute to plant nutrient acquisition, or are only passively arising in response to other, direct tolerance mechanisms affected by those efficient genotypes. The lack of studies in rice that contrast different genotypes is one area of research that needs attention.
An alternative to breeding cultivars with an inherent ability to foster indigenous, favourable microbial communities would be to select plant genotypes for compatibility with known plant growth-promoting microorganisms. Microorganisms such as plant-growth-promoting rhizobacteria enhance the availability of P through solubilization of soil-bound P, mainly through the release of organic acids such as gluconic acid, or by chelating cationic partners of P, thereby releasing P into soil solution (Goldstein et al., 1993). It is well established that many rhizosphere microorganisms can stimulate plant P acquisition (reviewed by Richardson et al., 2011) and Zn acquisition (e.g. Tariq et al., 2007) under laboratory and glasshouse conditions, but their contribution to plant nutrition in the field is somewhat inconsistent (Lucy et al., 2004). For example, inoculation of rice with a mixed-strain inoculant containing four different plant-growth-promoting microorganisms, one of which can solubilize P through acidification and phytase production (Amprayn et al., 2012), did not improve P-use efficiency under field conditions (Cong et al., 2010) but did improve overall growth and N-use efficiency (Cong et al., 2009).
In summary, there is little concrete evidence to suggest that exploiting rhizosphere microbes will confer greater root acquisition of P and Zn, but this may simply reflect our poor understanding of what is an extremely complex area rather than lack of scope for exploitation. Given the large increases in rice root efflux of sugars, amino acids, LMWOAs and other compounds under both P and Zn deficiency (Suzuki et al., 2009; Rose et al., 2011a), we speculate that greater understanding of rhizosphere microbial dynamics may lead to opportunities for enhancing the root acquisition of Zn and P over the coming decades.
ROOT TRAITS TO INCREASE SOIL EXPLORATION
Because both P and Zn are immobile in soil, the majority of uptake of these nutrients will be driven by diffusion rather than mass flow and, therefore, root exploration into new volumes of soil will be critical (Barber, 1984). Further, the distribution of P and Zn are not uniform across a soil profile and their concentrations tend to be highest in the upper soil horizons, and may be patchy (Ma et al., 2009). Thus, an ideal deficiency-tolerance mechanism would include thorough exploration of those regions of the profile where P or Zn is most abundant.
Root architecture
In upland fields, root systems that maximize the spatial exploration of P-rich soil zones with minimal overlap of root depletion zones is likely to enhance P and Zn acquisition. Increased lateral and adventitious rooting and shallower growth of seminal roots (or basal roots in the case of dicots) appear to confer enhanced genotypic P acquisition in other cereal crops (Lynch, 2007). Rice, however, is relatively susceptible to water deficit, and there is likely to be a trade-off between topsoil foraging root traits and traits that enable greater water acquisition from deeper in the soil profile (Ho et al., 2005). It is therefore unclear whether topsoil foraging traits beneficial in other crop species provide overall benefits for rain-fed lowland- and upland-rice crops. Given that rain-fed rice systems are generally drought prone, it will be important to develop rice that combines both efficient water uptake and nutrient foraging.
Good progress has recently been made with the development of drought-tolerant rice-breeding materials (Dixit et al., 2012) and the identification of a quantitative trait locus (QTL) for steep root angles, Deep root (Dro1) (Uga et al., 2011), is expected to lead to further advances in drought tolerance. Given the importance of drought, it is therefore unrealistic to contemplate breeding for purely shallow roots. The only way would be to establish that shallow and deep roots are under independent genetic control, which would allow for the development of cultivars with a sufficiently large proportion of deep roots while concentrating the remaining roots in shallower, nutrient-rich regions. That this may indeed be possible is suggested by the observation that the main QTL for shallow rooting, qSOR1 (Soil Surface Rooting 1; Uga et al., 2012) was mapped to chromosome 7 while Dro1 is located on chromosome 9 (Uga et al., 2011). The attempt to develop rice cultivars with such bi-modal rooting patterns is now in an initial stage, relying on the introgression of the Dro1 and qSOR1 loci into a common recipient variety such as ‘IR64’. It remains to be seen whether qSOR1 can indeed enhance P uptake as expected.
Optimum branching patterns and deployment of roots for P and Zn acquisition are likely to differ for rice grown in flooded versus upland/rain-fed fields. In particular, the importance of basal roots and topsoil exploration in flooded soils has not been resolved. Topsoils of flooded rice paddies may not be richer in P as in aerobic soils since paddy fields do not have a structured soil profile due to repeated puddling. In addition, the upper few centimetres of a flooded soil tend be oxic compared with the reduced soils deeper down (Kirk, 2004) and, as such, the presence of an oxidized layer may lead to decreased P availability or an increased Zn availability compared with the lower parts of the soil profile.
Potentially in flooded rice soils, the configuration of root systems may be most efficient when they maximize the re-capture of solubilized P or Zn, rather than root systems that maximize exploration of the soil volume or explore the topsoil layers. Studies with Zn-efficient rice lines revealed that forming hills with a greater number of plants led to increased Zn nutrition of crops (Hoffland et al., 2006). While this may be the result of increased localized concentrations of root exudates to solubilize Zn, is it also possible that the intertwined roots actually enabled greater capture of Zn because, while the Zn-chelate complex may diffuse away from the root that released the chelating agent, the presence of neighbouring roots ensures a high chance of re-capture. The latter hypothesis has been supported by a recent modelling study which found that root length density was the parameter with the greatest influence on Zn uptake when Zn was solubilized and chelated by the phytosiderophore DMA (Ptashnyk et al., 2011). Essentially, because diffusion rates are higher in flooded soil, overlapping root diffusion zones are highly beneficial because roots can acquire Zn complexed with chelates released from neighbouring roots (Ptashnyk et al., 2011).
As with root traits that aid solubilization of P/Zn in the rhizosphere, there have been limited physiological and genetic studies on rice root architecture, and modelling has been heavily relied upon. Kirk (2003) investigated the trade-off involved in root requirements for internal aeration and optimal characteristics for nutrient uptake, and the model developed predicted that the current structure of rice roots, whereby a coarse aerenchymous primary root contained numerous fine, short lateral roots, would be the optimum combination to satisfy the needs for nutrient uptake and root aeration. Modelling studies on Zn acquisition by lowland rice suggested that the half distance between root axes was a critical parameter in determining Zn uptake (Adhikari and Rattan, 2000); however, evidence from field studies to support this theory is currently lacking.
Fine structure to increase root surface area at low cost
In the absence of soil heterogeneity, P and possibly Zn uptake is typically thought to be proportional to the root surface area developed by a cultivar. Since the development of root biomass represents a cost to the plant, both in terms of carbon and P, any adaptation that reduces the carbon or P costs to the plant would be beneficial. A number of root traits that increase the surface area for P absorption at a low metabolic cost have been identified in crop plants, including the formation of finer roots, development of aerenchyma and the formation of root hairs (Lynch, 2007). The formation of root hairs and the ability to produce longer and finer roots has also been linked to enhanced Zn uptake in other dryland cereal crops such as wheat and barley (Dong et al., 1995; Genc et al., 2007).
Rice plants also respond to P deficiency by increasing the length and density of root hairs (Fig. 2). The response appears to be mediated by both the internal-shoot P concentration and the soil-solution P concentration at the root surface (Fig. 2). However, the data were obtained from experiments with ‘Nipponbare’, a cultivar with poor tolerance to P deficiency, which suggest that the root hair response is a common response of rice to P deficiency and it remains to be seen whether scope exists to increase root-hair length and density beyond the level already present in standard varieties like ‘Nipponbare’.
Fig. 2.
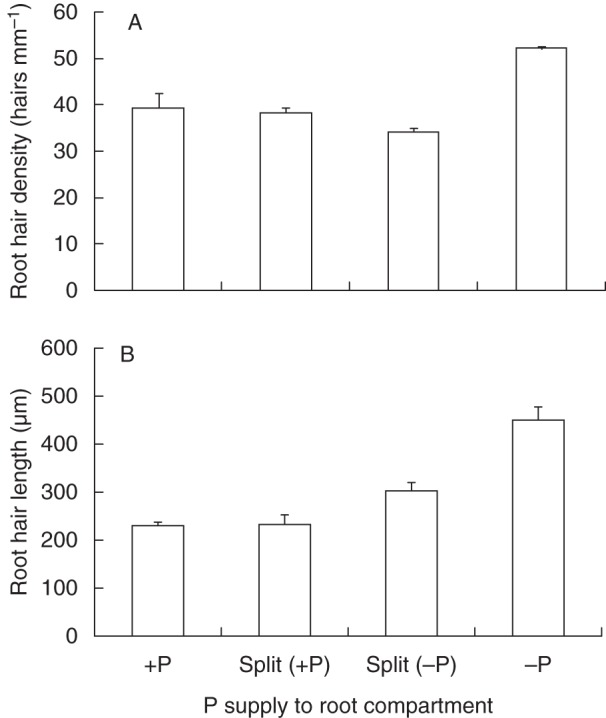
The impact of P supply to roots on (A) root hair density and (B) length of the P-inefficient rice cultivar ‘Nipponbare’ in a split-root study. Rice seedlings were raised on a floating mesh for 7 d before being transplanted into 90 mm × 90 mm × 200-mm-deep pots which contained P-deficient andosol soil (described in Wissuwa and Ae, 2001a). Compartments were either watered with full-strength Yoshida nutrient solution (+P) or Yoshida solution without P (–P) and plants were harvested after 37 d. The split-root treatment creates plants that have adequate shoot-tissue P concentrations but whose roots are exposed to P-replete conditions in one root compartment but P-deficient conditions in the other. Root growth/physiology in each compartment therefore reflects plant response to the external root environment only. Root hairs were measured microscopically using an Olympus BX50 equipped with a DP20 digital camera (Olympus Imaging, Tokyo, Japan). Root-hair length was measured on digital micrographs of 1st order lateral roots, using segments of root 0–30 mm from the root tip. Photographs were taken at 0·2, 0·7, 1·2, 1·7 and 2·2 mm from the tip of six root segments and the five longest hairs per slide were measured and averaged.
Increased root fineness and enhanced root aerenchyma formation are known P stress responses in rice (Kirk and Le Van Du, 1997), which suggests that such mechanisms may play a role in efficient acquisition of P. These traits would also, in theory, benefit Zn acquisition due to enhanced root exploration of the soil volume at a low metabolic cost. The key question with any of the above traits remains whether there is demonstrable genotypic variation for these traits linked to P/Zn acquisition of rice in the field that can be exploited through plant breeding. This question is currently being assessed using a genome-wide association mapping approach based on the diversity present in 400 rice Genebank accessions encompassing a very large portion of the rice gene pool (http://www.ricediversity.org/people/collaborators.cfm; also see the section ‘Breeding strategies and future prospective’).
An alternative to relying entirely on roots for P uptake is for plants to form associations with mycorrhizal fungi. This subject has been widely studied and reviewed (see Smith and Smith, 2011), but because infection of rice roots by mycorrhizal fungi is rare in flooded soils there have been relatively few studies on mycorrhizal colonization of rice. Aerobic inoculation of arbuscular mycorrhizal fungi (AMF) (Glomus sp.) in the nursery of rice plants showed around 50 % survival after transplantation to flooded fields but the inoculation of AMF directly in the flooded paddy was not successful (Solaiman and Hirata, 1997, 1998). Further studies using different strains of AMF over a number of genotypes are necessary to understand the role of mycorrhizae in Zn uptake in flooded paddy fields before concluding whether or not mycorrhizae play a significant role in Zn or P uptake by rice plants under flooded conditions.
In contrast to Zn, there is evidence that the presence of mycorrhizae enhances P uptake in upland rice crops (Maiti et al., 2011a, b). Further, the fact that arbuscular mycorrhizae (AM) specifically induce the rice high-affinity P transporter OsPT11 (Paszkowski et al., 2002) suggests that AM fungi may play a specialized role in P acquisition in upland rice. Gao (2007) also reported that inoculation with AM improved Zn uptake of aerobic rice, but only in genotypes with inherently low Zn uptake. It was thus concluded that breeding rice plants with a high capacity to solubilize Zn in conjunction with responsiveness to AM colonization was unlikely to be feasible (Gao, 2007).
We are not aware of any evidence that AM colonization is linked to genotypic Zn or P efficiency in rice. The fact that AM colonization has not been successfully exploited in the breeding programmes of other upland crops suggests that scope for exploiting it in rice breeding is limited.
Maintenance of crown root development
Phosphorus deficiency typically leads to an increase in the root : shoot ratio and while this may involve an absolute increase in root biomass under mild P deficiency, more severe P or Zn deficiency typically leads to an absolute decrease in root biomass (Wissuwa and Ae, 2001a, b) and the increasing root : shoot ratio would thus be due to a proportionally larger decline in shoot biomass. This decrease in root biomass was attributed to a direct effect of low P availability rather than to a lack of assimilate supply from shoots (Wissuwa et al., 2005). In rice almost the entire root system is derived from crown roots that continuously emerge from the lower nodes of the shoot. The rate at which these crown roots emerge is sensitive to stress, and Zn-deficiency was found to reduce crown-root number by up to 75 % in a sensitive cultivar, while a tolerant cultivar maintained a much higher proportion of the non-stress crown root number (Widodo et al., 2010; see also Fig. 3A).
Fig. 3.
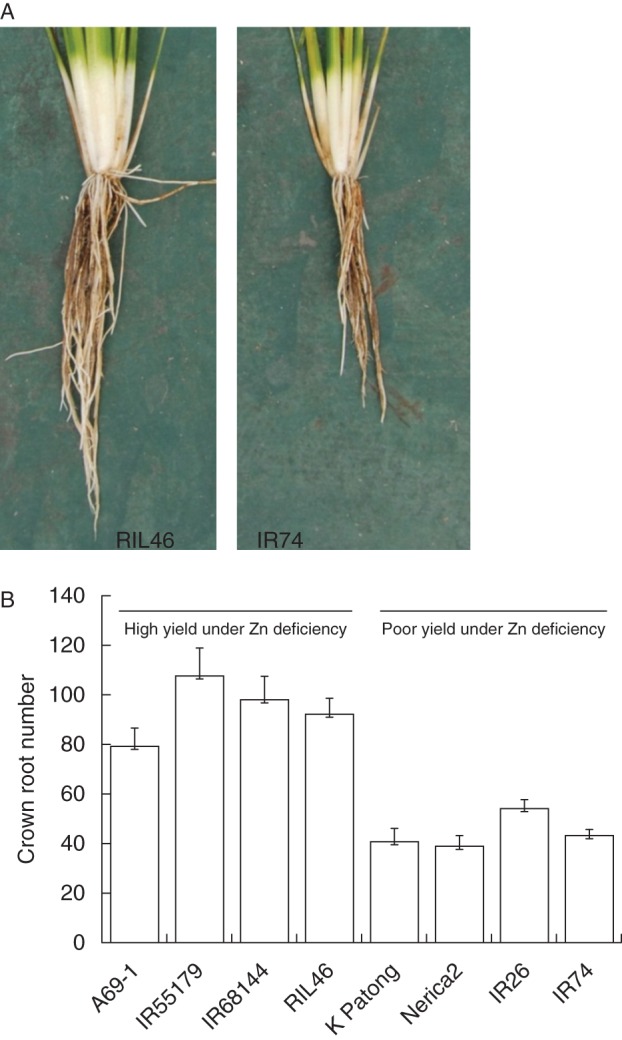
Careful removal of entire rice plants from a Zn-deficient soil show differences in root system size between a tolerant (RIL46) and sensitive (IR74) genotype (A). Crown-root number of field-grown rice seedlings were sampled 3 weeks after transplanting into a Zn-deficient field (B). In repeated field screening trials, lines A69-1, IR55179, IR68144 and RIL46 had been identified as highly tolerant of Zn deficiency, whereas the four lines on the right represent intolerant varieties.
Recent data showed that this ability to maintain crown root development under Zn deficiency is a general feature shared by all cultivars possessing tolerance to Zn deficiency (S. M. Impa, IRRI, Los Baños, The Philippines, unpubl. res; Fig. 3B). That the difference in crown root emergence can be detected as early as 3 d after transplanting implies that this trait is an independent tolerance mechanism. As such, it is now one of the main traits targeted in breeding for enhanced tolerance to Zn deficiency, and efforts are under way to map loci controlling the trait (G. Gregorio, IRRI, Los Baños, The Philippines and M. Wissuwa, JIRCAS, Tsukuba, Japan, unpubl. res.). Studies characterizing the effect of the Pup1 locus on plant performance under P deficiency also hint to an involvement of Pup1 in maintaining crown root emergence (Gamuyao et al., 2012), further highlighting the potential of this trait in rice breeding for nutrient-poor environments.
Root traits to increase uptake efficiency: P and Zn transporters
Plants take up orthophosphate from the soil via phosphate transporters to overcome the steep P concentration gradient between the soil solution and the cytoplasm of root cells (Raghothama, 1999). One adaptation of plants to P deficiency is the up-regulation of high-affinity P transporters in the roots, giving rise to the hypothesis that manipulation of these transporters at the molecular level may improve the tolerance of plants to low-P soils (Muchhal and Raghothama, 1999). A number of attempts have been made in other upland crops to improve P nutrition by manipulating the expression of such transporters, but these studies have yielded little success to date (reviewed by Richardson et al., 2009). For example, transgenic barley plants that overexpressed a P transporter failed to acquire more P from nutrient solution or soil than wild-type plants (Rae et al., 2004). Plants seem well adapted for obtaining P from the low concentrations in soil solution, and the movement of P into the soil solution from solid phases may be the rate-limiting step.
Rice has 13 known P transporters, of which 12 are expressed, and an additional 13 putative P transporters have recently been identified (Liu et al., 2011). So far, only two of these transporters have been functionally characterized, revealing that OsPT2 encodes a low-affinity and OsPT6 a high-affinity transporter (Ai et al., 2009). To date, our studies on P transporters in rice have led to similar conclusions to those of Richardson et al. (2009). Up-regulation of P transporters in root tissue of the P-inefficient rice cultivar ‘Nipponbare’ occurred at similar levels to those observed in the P-efficient NIL6-4 under P deficiency (Pariasca-Tanaka et al., 2009), suggesting that even cultivars that are poorly adapted to P-deficiency have well-adapted P transporters in roots. Similar data were obtained from a study using the irrigated rice variety ‘IR64’ (Gamuyao et al., 2012). Our studies investigating the Michaelis–Menten kinetics of rice genotypes that differ in P efficiency in upland fields have found that higher Km values or lower Cmin values are not specifically associated with P-efficient or P-inefficient lines (Table 1). However, it is worth noting that large-scale screening experiments that specifically target root P-uptake rates have not yet been conducted.
Table 1.
Kinetic P-uptake parameters (initial [P] = 20 µm) for six genotypes previously grown under P-deficient or P-replete hydroponic conditions
| Pre-treatment | Genotype (tolerance to P deficiency)* | Maximum uptake rate, Imax (μmoles g−1 h−1) | Concentration at half Imax, Km (μm) | Minimum concentration, Cmin (μm) | Maximum uptake rate/efflux rate (Imax/X) |
|---|---|---|---|---|---|
| –P | Kasalath (E) | 13 ± 2 | 1·7 ± 0·6 | 0·22 ± 0·06 | 5·3 ± 2·0 |
| Nerica10 (E) | 8 ± 3 | 1·5 ± 1·1 | 0·15 ± 0·04 | 6·5 ± 0·3 | |
| IAC47 (E) | 26 ± 15 | 7·8 ± 3·9 | 0·19 ± 0·01 | 15·5 ± 4·7 | |
| Dular (E) | 32 ± 12 | 7·1 ± 4·0 | 0·16 ± 0·02 | 17·7 ± 5·3 | |
| Nipponbare (I) | 19 ± 2 | 2·6 ± 0·7 | 0·23 ± 0·07 | 4·1 ± 0·6 | |
| Nerica9 (I) | 28 ± 12 | 6·5 ± 4·1 | 0·21 ± 0·03 | 13·5 ± 7·6 | |
| + P | Kasalath (E) | 6 ± 1 | 1·2 ± 1·0 | 1·3 ± 1·2† | 1·9 ± 0·1 |
| Nerica10 (E) | 8 ± 1 | 1·6 ± 1·6 | 1·3 ± 1·6 | 2·2 ± 0·1 | |
| IAC47 (E) | 8 ± 2 | 2·4 ± 2·1 | 0·6 ± 0·2 | 4·6 ± 2·0 | |
| Dular (E) | 5 ± ± 1 | 1·7 ± 0·3 | 1·6 ± 0·1 | 2·2 ± 0·4 | |
| Nipponbare (I) | 24 ± 17 | 3·5 ± 2·8 | 0·6 ± 0·3 | 2·8 ± 0·7 | |
| Nerica9 (I) | 11 ± 1 | 1·5 ± 0·4 | 0·6 ± 0·1 | 3·4 ± 0·9 |
* The genotypes are categorized as P-efficient (P) or P-inefficient (I), based on tolerance rankings under field conditions.
† Values in bold given under +P conditions represent the concentration in solution after 28 h, at which time it is likely that depletion had not yet ceased.
While it appears that prospects for improving P uptake through higher expression of root P transporters are limited in upland rice, the case may be different for lowland rice because of differences in soil chemistry. Under flooded soil conditions, diffusion coefficients for P are predicted to be 10–100 times faster because of the reduced tortuosities of diffusion paths (Turner and Gillam, 1976). As a consequence, the uptake of P across the root membrane may be the rate-limiting step for P acquisition, rather than speed of replenishment of the soil solution P (i.e. solution–solid phase P equilibria). It must be borne in mind that other conditions could moderate this effect, such as the accelerated formation of Fe-compounds in reduced soils increasing P-binding sites (Kirk, 2004). We are unaware of any direct evidence to support the dominance of one process over the other and, therefore, whether solid-solution phase P equilibria are more rapid in flooded versus aerobic soils. This aside, enhanced P uptake by rice plants in low-to-medium-P flooded soil has recently been reported by overexpression of a P transporter OsPht1;8 (OsPT8) (Jia et al., 2011; Wu et al., 2011). Given that OsPT8 was expressed in a range of tissues other than roots, it is possible that overexpression of this transporter (or overexpression of OsPHR2) somehow altered the partitioning of P within the plant, which may have enabled greater root growth to acquire P from the soil. However, in the absence of any evidence of this in the studies of Jia et al. (2011) and Wu et al. (2011), the logical conclusion to draw would be that overexpression of the OsPT8 resulted in a greater density of P transporter proteins per unit of root length, which enabled higher P uptake rates from the soil solution. The results of Jia et al. (2011) and Wu et al. (2011) are, on face value, quite promising; however, until further understanding of the mechanism(s) of higher P uptake is gained and verification of results over a wider range of soils is obtained, it is difficult to speculate how useful such a trait may be.
Plants take up Zn from the soil using transporters from the Zn-regulated transporters in the iron (Fe)-regulated transporter-like protein (ZIP) gene family. ZIP transporter genes and their known or putative roles in rice plants have been recently reviewed by Ishimaru et al. (2011), and interested readers are encouraged to read that review for detailed discussions on Zn transporters in rice. Essentially, while several root-expressed ZIP transporter genes are up-regulated under Zn-deficiency stress (Ishimaru et al. 2011), there is no evidence yet to suggest that Zn-efficient rice genotypes have greater expression of particular Zn transporters in roots, or that overexpression of ZIP transporter genes in roots confers greater Zn uptake in Zn-deficient soils. Further, studies on Zn uptake kinetics have also concluded that while high-affinity Zn uptake systems exist, they do not appear to be involved in any genotypic tolerance to Zn deficiency in upland crops such as wheat (Hacisalihoglu et al., 2001) or in lowland rice (Bowen, 1986).
WHOLE-PLANT TRAITS TO IMPROVE INTERNAL EFFICIENCY AND MAINTENANCE OF ROOT TRAITS
Resource allocation between root and shoot
It is expected that plants tightly regulate and balance processes representing a cost to the plant, such as developing and maintaining roots, entering symbiotic relationships with mycorrhizae, or releasing nutrient-solubilizing agents from roots (Lambers et al., 2006). In experiments varying light level and P supply, it was shown that P deficiency generally led to an increase in the root : shoot ratio (from below 20 % to 40 %) even under low-light conditions, due mainly to a decrease in shoot biomass while root biomass was kept constant or declined slightly under more severe P deficiency (Wissuwa et al., 2005). In their study, Wissuwa et al. (2005) detected increasing starch concentrations with increasing P deficiency in roots, but much less so in shoots, which suggested that (a) assimilates were preferentially distributed to roots and (b) that assimilate supply may not have been the growth-limiting factor. Instead it was concluded that low P availability directly limited growth and that the distribution of P between root and shoot (the root : shoot P ratio) was an important factor. It increased from 20 % to 30 % under P deficiency and in combination with a very high internal P-use efficiency in roots, contributed to the overall increasing root : shoot ratio for biomass. We have recently shown that internal P-use efficiency differs among rice genotypes (Rose et al., 2011b; Rose and Wissuwa, 2012), and further work in characterizing promising genotypes is ongoing.
If P is indeed directly limiting plant growth under more severe P deficiency, distributing a bigger proportion of assimilates to roots to maintain root growth or to enhance root exudation may not represent a critical cost factor for plants. Furthermore, where P directly limits growth, fine-tuning the distribution of it will be very crucial. In fact, model simulations suggested that a small increase in the root : shoot P ratio would be one of the most efficient ways to overcome P deficiency since the extra P in roots would drive root growth and further P uptake (Wissuwa, 2003). In that regard the low-cost root system discussed in the subsection ‘Fine structure to increase root surface area at low cost’ may have to be seen primarily from the point of low cost of P, not carbon.
Recent analyses of rice root exudates by GC-MS (gas chromatography–mass spectrometry) show that a myriad of different sugars, amino acids and secondary metabolites leave the rice root along with the more conventionally-measured LMWOAs citrate and malate (Suzuki et al., 2009; Tawaraya et al., 2009). Unfortunately precise calculations of the total carbon being thus exuded are still lacking but it appears that, accounting for all other metabolites, the total carbon being lost from roots could be 10–50 times greater than the amount of citrate/malate exuded. A carbon loss of such magnitude could indicate that (a) growth under P deficiency is indeed not limited by carbon and that (b) the seemingly unspecific exudation of a multitude of compounds many perform a beneficial function in the rhizosphere, possibly by enhancing plant–microbe interactions in some way that may eventually provide better access to P to the rice plant.
In light of the potentially unresolved question of carbon or P limitations under P deficiency it would be desirable to model the effect of alternative root-to-shoot distribution patterns of P and carbon on the growth and nutrient acquisition of rice under various levels of P deficiency. This approach may provide a different set of optimum solutions, both in terms of P and carbon allocation for plant growth or of carbon allocation for root exudation, depending on the severity of P deficiency. Such modelling exercises would not only improve our understanding of the plasticity of plant responses to P but may ultimately be of use in defining selection criteria for plant breeding. Unfortunately it appears that most current plant growth models are ill-suited to perform such a task because growth is typically modelled as a function of carbon assimilation (mainly affected by radiation interception, temperature and possibly water and N uptake) but not as a function of other growth-limiting factors.
Overcoming oxidative stress
Both macronutrient and micronutrient deficiencies in plants induce an oxidative stress that manifests as higher-than-usual levels of reactive oxygen species (ROS), increased lipid peroxidation and protein oxidation, loss of cell integrity and, eventually, cell death and tissue decay (Gill and Tuteja, 2010). If root damage by ROS can be minimized it would be expected that the maintenance of root function would further contribute to overcoming deficiency through the continuation of soil exploration and nutrient acquisition.
Under P deficiency, oxidative stress in the form of increased H2O2 production and lipid peroxidation is generally considered to be mild (Malusa et al., 2002). In contrast, Zn deficiency in plants usually results in severe cell damage by ROS, mainly because Zn is a necessary component in numerous antioxidant and homeostatic enzymes (Broadley et al., 2007). Indeed, increased ROS is considered to be the primary mechanism by which Zn deficiency damages crop plants (Cakmak, 2000). Furthermore, oxidative damage is strongly exacerbated by high concentrations of soil bicarbonate that commonly co-occur with Zn deficiency (Rose et al., 2011a). Gill and Tuteja (2010) propose that abiotic stress tolerance may therefore be improved by enhancing in vivo levels of antioxidant enzymes or metabolites in plants. Recent studies on Zn-efficient rice genotypes shows that levels of ROS and ROS-related damage are consistently lower than in inefficient genotypes, and that this tolerance is often correlated with increased levels of antioxidant enzymes or metabolites (Table 2). Such an investment in greater ROS-defence mechanisms may come at a slight biomass- or yield-cost under highly productive conditions; however, under Zn-deficient (and possibly P-deficient) environments, breeding for such traits would appear to be desirable.
Table 2.
Summary of the ROS and antioxidant levels of Zn-efficient and Zn-inefficient genotypes under Zn-deficiency stress
| Study | Genotypes | Plant tissue | ROS levels (efficient vs. inefficient) | Antioxidant enzyme or metabolite levels (efficient vs. inefficient) |
|---|---|---|---|---|
| Chen et al. (2008) | Zn-efficient genotype: IR8192 | Shoot | Lower cell membrane permeability | Higher total SOD |
| Higher POD | ||||
| Zn-inefficient genotype: Erjiufeng | Lower MDA | No difference CAT | ||
| Lower H2O2 | No difference APX | |||
| Hajiboland and Beiramzadeh (2008) | Zn-efficient genotype: Amol | Shoot | Higher H2O2 | No difference total SOD |
| No difference MDA | No difference POD | |||
| Zn-inefficient genotype: Dashti | No difference CAT | |||
| No difference APX | ||||
| Root | No difference H2O2 | No difference total SOD | ||
| No difference MDA | Higher POD (in aerated hydroponics) | |||
| No difference superoxide | No difference CAT | |||
| No difference APX | ||||
| Chen et al. (2009) | Zn-efficient genotype: IR8192 | Root | Lower cell membrane permeability | Higher total SOD |
| Higher POD | ||||
| Zn-inefficient genotype: Erjiufeng | Lower MDA | No difference CAT | ||
| Lower H2O2 | No difference APX | |||
| Frei et al. (2010) | Zn-efficient genotype: RIL46 | Shoot | Lower H2O2 | No difference total SOD |
| Zn-inefficient genotype: IR74 | Lower superoxide | Lower POD | ||
| No difference CAT | ||||
| Lower APX | ||||
| Higher ascorbic acid (total and reduced) | ||||
| Rose et al. (2012) | Zn-efficient genotype: RIL46 | Root | Lower H2O2 | No difference total SOD |
| Higher non Cu/Zn SOD | ||||
| Zn-inefficient genotype: IR74 | No difference POD | |||
| No difference APX | ||||
| Higher gluconic acid | ||||
| Higher oxolutaric acid |
Abbreviations: MDA, malondialdehyde; SOD, superoxide dismutase; POD, peroxidase; CAT, catalase; APX, ascorbate peroxidase.
General response to stress: stress response versus tolerant response
One result of microarray and metabolomic comparisons between tolerant and sensitive cultivars typically has been that stress-response pathways and genes are expressed at a higher level in sensitive cultivars, while tolerant ones maintain normal plant functions to a higher degree (Pariasca-Tanaka et al., 2009; Widodo et al., 2010; Rose et al., 2012). Physiological studies also indicate that tolerance to Zn deficiency by some rice genotypes is conferred by the capacity to continue the efflux of DMA, rather than reduce its efflux, as is observed in Zn-intolerant lines (M. T. Rose, unpubl. res.). It cannot be ruled out that these differences are due to lower stress levels being experienced by tolerant cultivars as a result of better nutrient uptake. However, it is also possible that some sensing/regulation event tells plants to behave differently, and that the ‘stress response’ (referred to in the literature in the case of P deficiency as ‘the Pi deprivation response’) is not beneficial. Perhaps the best-documented evidence of this is the PHO1 response in arabidopsis reported by Rouached et al. (2011). The PHO1 gene in arabidopsis (AtPHO1) is involved in translocation of Pi from roots to shoots, and PHO1 mutants show P-deficiency symptoms in shoots when plants are grown in P-replete conditions (Morcuende et al., 2007). Rouached et al. (2011) showed that arabidopsis and rice plants with reduced expression of the PHO1 gene sustained growth when transferred to a P-deficient medium (to the level of plants grown in a P-replete medium), while wild-type plants showed a marked decrease in growth upon transfer to the P-deficient growing medium. The plants with reduced PHO1 expression were able to grow because there was sufficient Pi in vacuoles that was remobilized for metabolic functions, where in wild-type plants the typical Pi-deprivation response was triggered, causing a reduction in growth rate despite there being sufficient Pi stored in the vacuoles to sustain normal growth (Rouached et al., 2011). Essentially, suppressing the Pi deprivation response allowed plants to sustain growth. We hypothesize that many genes that confer root traits which overcome abiotic stresses may in fact be those that avoid the typical stress-response pathways.
BREEDING STRATEGIES AND FUTURE PROSPECTIVE
Breeding for improved nutrient acquisition can be approached with modern breeding tools, based on transgenic approaches or MAS, or through conventional breeding schemes that typically rely on some form of phenotypic selection in target environments. In each case a breeder will need to decide whether to base their selection on some trait thought to improve nutrient uptake and yield, or whether to follow a purely performance-based approach, selecting for yield (grain, biomass) or nutrient uptake. In this final section we will discuss the pros and cons of these alternative approaches.
Performance-based selection
Screening experiments on nutrient-deficient soils typically expose varietal differences in grain yield or total nutrient uptake (Wissuwa and Ae, 2001a; Quijano-Guerta et al., 2002) and we can assume that this variation is, in part, due to some past selection in a similarly nutrient-limited environment. Thus, straightforward selection for performance under nutrient deficiency has worked in the past and will continue to produce superior cultivars in the future. In fact the IRRI breeding line IR55179 (see Fig. 3B) serves as a good example, since it was recently selected from trials on low-Zn soils where it out-performed even the most tolerant check variety (S. M. Impa and G. Gregorio, IRRI, Los Baños, the Philippines, unpubl. res.).
One potential complication of conventional breeding for improved nutrient acquisition is that nutrient deficiencies tend to be patchy across field plots. Heterogeneous nutrient availability at screening sites can result in environmental effects masking genetic effects and, as a result, the realized heritability may be rather low. To improve the gains made in selection may require very extensive and costly field testing in multiple target environments (Wissuwa et al., 2009). This potential drawback of performance-based conventional selection may be overcome by the use of modern breeding approaches that rely on molecular markers tightly linked to loci controlling field performance. MAS is particularly suitable for traits with low heritability because the need for extensive field screening is reduced, since lines of a breeding population that do not have the desired marker genotype can be eliminated before field evaluations.
The prerequisite for employing MAS in the improvement of nutrient acquisition in rice obviously is the availability of markers tightly linked to a locus or gene significantly enhancing nutrient uptake under field conditions. Marker availability is no longer a limiting factor in rice because the rice genome sequence offers near limitless opportunities for marker design – instead the bottleneck in applying MAS lies in the low number of loci that have been mapped with the required precision and have been confirmed to affect plant performance in the field. At present the best example of MAS for a nutrient-acquisition trait in rice is the Pup1 locus, which had been mapped as a major QTL for P uptake, biomass and grain yield under low-P field conditions (Wissuwa and Ae, 2001a; Wissuwa et al., 2002). Markers diagnostic of the Pup1 locus in a wide variety of rice cultivars have been developed (Chin et al., 2010, 2011) and Pup1 has now been transferred to modern rice variation through marker-assisted backcrossing, showing first encouraging results in field screening trials (Gamuyao et al., 2012).
Trait-based selection
Much of the work in plant nutrition has been devoted to the identification of traits thought to enhance nutrient uptake and we have reviewed those linked to P and Zn uptake in preceding sections. Despite the attention received, few traits have proved effective in applied plant breeding, the selection for shallow root angle being one of the few positive exceptions (Lynch, 2007; Wissuwa et al., 2009). Before specific traits can be considered in selection, whether in conventional breeding or MAS, they need to meet two requirements, namely that (a) genotypic variation exists within the rice gene pool for the gene(s) controlling the trait, and (b) this genotypic variation leads to an advantage in nutrient uptake under field conditions. Unfortunately there are few examples that meet both requirements and an urgent need therefore exists to identify those traits that have a plausible chance of meeting these requirements. For example, while P or Zn transporters are no doubt crucially important for P and Zn uptake, there is no evidence suggesting that genotypic variation exists that could be exploited in breeding. Transgenic approaches that alter the expression of key genes are one potential solution to the lack of genotypic variation; however, until now most attempts to enhance plant performance have not been successful (Wissuwa et al., 2009; Richardson et al., 2011). Whether the transgenic approach becomes increasingly relevant for nutrient uptake traits in the future will depend not only on the identification of novel key genes but equally on the public acceptance of transgenic staple crops like rice.
Conclusions and outlook
In the case of some traits that have been clearly implicated in Zn or P efficiency in rice such as DMA efflux or ROS tolerance (Fig. 1), it may be prudent to undertake genotypic screening trials that specifically target these traits, followed by classical QTL mapping using bi-parental crosses made based on the initial screening experiments. Such an approach has proved useful in identifying QTLs for root shallowness which confers P-efficiency in the field and might be useful for breeding (Lynch, 2007). However, we believe a more promising approach is the classical forward genetic approach that typically starts with the identification of a donor with superior performance in a target environment, followed by QTL mapping and eventual cloning of the underlying gene. Forward genetics has the advantage that it is not based on pre-conceptions of what trait can enhance nutrient acquisition and therefore is not limited by our incomplete current knowledge. Furthermore, forward genetics enables the discovery of new tolerance mechanisms and genes as exemplified by the Pup1 major gene that is a novel gene not present in the rice reference genome and would therefore have been impossible to detect by other strategies (Gamuyao et al., 2012). This phenomenon has also occurred for other abiotic stress-tolerance traits in rice, such as submergence tolerance (Sub1) (Xu et al., 2006).
Given the central role of P and Zn in metabolic and catabolic processes, and the complexity of soil–plant interactions, a potential limitation of forward genetics appears is that multiple loci/genes interact to produce a superior phenotype and that the detectable contribution of each locus to the overall phenotypic variation within a population remains too small (Widodo et al., 2010) for breeders to be convinced of their utility in MAS. In such cases a trait-based approach may offer advantages if combined with a forward genetics strategy. Identifying the main tolerance mechanisms segregating in mapping populations, followed by QTL mapping of these tolerance mechanisms, possibly under highly targeted artificial screening conditions, should increase the power of detecting major QTL. To establish their relevance it would have to be shown that such trait-specific major QTL co-localize with QTL detected for field performance. At present this strategy is being employed to identify major QTL for tolerance to Zn deficiency – the two main tolerance mechanisms used for trait-based QTL mapping being crown root emergence and DMA exudation (see subsections ‘Efflux of ligands from roots’ and ‘Maintenance of crown root development’).
In the past QTL mapping has been a rather lengthy process, often taking 10 years or more, but new high-throughput marker platforms promise to speed up conventional QTL mapping, while genome-wide association studies may offer opportunities to even bypass some of the steps needed in QTL mapping. Furthermore, mapping based on genome-wide association studies using association panels of several hundred rice genebank accessions would offer the additional advantage that the genotypic variation assessed is much wider compared with the traditional QTL mapping based on a bi-parental cross. These new technologies offer opportunities as never before to identify loci and traits enhancing nutrient acquisition and we can expect rapid progress to be made in this area in the near future. Whether these loci and traits will eventually become tools in practical breeding will depend largely on whether breeders are convinced of their positive effect in a farmer's fields. Linking gene identification and trait characterization to field performance will therefore remain as important as it has always been.
LITERATURE CITED
- Adhikari T, Rattan RK. Modelling zinc uptake by rice using a Barber–Cushman approach. Plant and Soil. 2000;227:235–242. [Google Scholar]
- Ai P, Sun S, Zhao J, et al. Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. The Plant Journal. 2009;57:798–809. doi: 10.1111/j.1365-313X.2008.03726.x. [DOI] [PubMed] [Google Scholar]
- Amprayn K, Rose MT, Kecskés M, Pereg L, Nguyen HT, Kennedy IR. Plant growth promoting characteristics of soil yeast (Candida tropicalis HY) and its effectiveness for promoting rice growth. Applied Soil Ecology. 2012;61:295–299. [Google Scholar]
- Arnold T, Kirk GJD, Wissuwa M, et al. Evidence for the mechanisms of zinc uptake by rice using isotope fractionation. Plant, Cell & Environment. 2010;33:370–381. doi: 10.1111/j.1365-3040.2009.02085.x. [DOI] [PubMed] [Google Scholar]
- Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Annual Review of Plant Biology. 2006;57:233–266. doi: 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- Barber SA. Soil nutrient availability: a mechanistic approach. New York, NY: John Wiley & Sons; 1984. [Google Scholar]
- Begg CBM, Kirk GJD, MacKenzie AF, Neue H-U. Root-induced iron oxidation and pH changes in the lowland rice rhizosphere. New Phytologist. 1994;128:469–477. doi: 10.1111/j.1469-8137.1994.tb02993.x. [DOI] [PubMed] [Google Scholar]
- Berg G, Smalla K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiology Ecology. 2009;68:1–13. doi: 10.1111/j.1574-6941.2009.00654.x. [DOI] [PubMed] [Google Scholar]
- Bowen JE. Kinetics of zinc uptake by two rice cultivars. Plant and Soil. 1986;94:99–107. [Google Scholar]
- Briones AM, Okabe S, Umemiya Y, Ramsing NB, Reichardt W, Okuyama H. Influence of different cultivars on populations of ammonia-oxidizing bacteria in the root environment of rice. Applied and Environmental Microbiology. 2002;68:3067–3075. doi: 10.1128/AEM.68.6.3067-3075.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. Zinc in plants. New Phytologist. 2007;173:677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- Cakmak I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytologist. 2000;146:185–205. doi: 10.1046/j.1469-8137.2000.00630.x. [DOI] [PubMed] [Google Scholar]
- Chen WR, Yang X, He ZL, Feng Y, Hu FH. Differential changes in photosynthetic capacity, 77 K chlorophyllfluorescence and chloroplast ultrastructure between Zn-efficient and Zn-inefficient rice genotypes (Oryza sativa) under low zinc stress. Physiologia Plantarum. 2008;132:89–101. doi: 10.1111/j.1399-3054.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- Chen WR, He ZL, Yang XE, Feng Y. Zinc efficiency is correlated with root morphology, ultrastructure, and antioxidative enzymes in rice. Journal of Plant Nutrition. 2009;32:287–305. [Google Scholar]
- Chin JH, Lu X, Haefele SM, et al. Development and application of gene-based markers for the major rice QTL Phosphorus uptake 1. Theoretical and Applied Genetics. 2010;120:1073–1086. doi: 10.1007/s00122-009-1235-7. [DOI] [PubMed] [Google Scholar]
- Chin JH, Gamuyao R, Dalid C, et al. Developing rice with high yield under phosphorus deficiency: Pup1 sequence to application. Plant Physiology. 2011;156:1202–1216. doi: 10.1104/pp.111.175471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong PT, Dung TD, Hien TM, et al. Inoculant plant growth-promoting microorganisms enhance utilisation of urea-N and grain yield of paddy rice in southern Vietnam. European Journal of Soil Biology. 2009;45:52–61. [Google Scholar]
- Cong PT, Dung TD, Hien NT, et al. Effects of a multistrain biofertilizer and phosphorus rates on nutrition and grain yield of paddy rice on a sandy soil in Southern Vietnam. Journal of Plant Nutrition. 2010;34:1058–1069. [Google Scholar]
- Cordell D, Drangert J, White S. The story of phosphorus: global food security and food for thought. Global Environmental Change. 2009;19:292–305. [Google Scholar]
- Dixit S, Swamy B, Vikram P, et al. Fine mapping of QTLs for rice grain yield under drought reveals sub-QTLs conferring a response to variable drought severities. Theoretical and Applied Genetics. 2012;125:155–169. doi: 10.1007/s00122-012-1823-9. [DOI] [PubMed] [Google Scholar]
- Dong B, Rengel Z, Graham RD. Root morphology of wheat genotypes differing in zinc efficiency. Journal of Plant Nutrition. 1995;18:2761–2773. [Google Scholar]
- Fageria NK, Wright RJ, Baligar VC. Rice cultivar evaluation for phosphorus use efficiency. Plant and Soil. 1988;111:105–109. [Google Scholar]
- Frei M, Wang Y, Ismail A, Wissuwa M. Biochemical factors conferring shoot tolerance to oxidative stress in rice grown in low zinc soil. Functional Plant Biology. 2010;37:74–84. [Google Scholar]
- Gamuyao R, Chin JH, Pariasca-Tanaka J, et al. The protein kinase OsPSTOL1 from traditional rice confers tolerance of phosphorus deficiency. Nature. 2012;488:535–539. doi: 10.1038/nature11346. [DOI] [PubMed] [Google Scholar]
- Gao X. Bioavailabilty of zinc to aerobic rice. The Netherlands: Wageningen University; 2007. PhD Thesis. [Google Scholar]
- Gao X, Zhang F, Hoffland E. Malate exudation by six aerobic rice genotypes varying in zinc uptake efficiency. Journal of Environmental Quality. 2009;38:2315–2321. doi: 10.2134/jeq2009.0043. [DOI] [PubMed] [Google Scholar]
- Genc Y, Huang CY, Langridge P. A study of the role of root morphological traits in growth of barley in zinc-deficient soil. Journal of Experimental Botany. 2007;58:2775–2784. doi: 10.1093/jxb/erm142. [DOI] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Goldstein AH, Rogers RD, Mead G. Mining by microbe. Bioresource Technology. 1993;11:1250–1254. [Google Scholar]
- Hacisalihoglu G, Kochian LV. How do some plants tolerate low levels of soil zinc? Mechanisms of zinc efficiency in crop plants. New Phytologist. 2003;159:341–350. doi: 10.1046/j.1469-8137.2003.00826.x. [DOI] [PubMed] [Google Scholar]
- Hacisalihoglu G, Hart JJ, Kochian LV. High- and low-affinity zinc transport systems and their possible role in zinc efficiency in bread wheat. Plant Physiology. 2001;125:456–463. doi: 10.1104/pp.125.1.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajiboland R, Beiramzadeh N. Growth, gas exchange and function of antioxidant defense system in two contrasting rice genotypes under Zn and Fe deficiency and hypoxia. Acta Biologica Szegediensis. 2008;52:283–294. [Google Scholar]
- Hajiboland R, Yang XE, Römheld V, Neumann G. Effect of bicarbonate on elongation and distribution of organic acids in root and root zone of Zn-efficient and Zn-inefficient rice (Oryza sativa L.) genotypes. Environmental and Experimental Botany. 2005;54:163–173. [Google Scholar]
- Hardoim PR, Andreote FD, Reinhold-Hurek B, Sessitsch A, van Overbeek LS, van Elsas JD. Rice root-associated bacteria: insights into community structures across 10 cultivars. FEMS Microbiology Ecology. 2011;77:154–164. doi: 10.1111/j.1574-6941.2011.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AF. Soil organic phosphorus: a review of world literature. Wallingford, UK: CAB International; 1987. [Google Scholar]
- Hartmann A, Schmid M, van Tuinen D, Berg G. Plant-driven selection of microbes. Plant and Soil. 2009;321:235–257. [Google Scholar]
- Hedley MJ, Kirk GJD, Santos MB. Phosphorus efficiency and the forms of soil phosphorus utilized by upland rice cultivars. Plant and Soil. 1994;158:53–62. [Google Scholar]
- Hinsinger P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant and Soil. 2001;237:173–195. [Google Scholar]
- Ho MD, Rosas JC, Brown KM, Lynch JP. Root architectural tradeoffs for water and phosphorus acquisition. Functional Plant Biology. 2005;32:737–748. doi: 10.1071/FP05043. [DOI] [PubMed] [Google Scholar]
- Hoffland E, Wei CZ, Wissuwa M. Organic anion exudation by lowland rice (Oryza sativa L.) at zinc and phosphorus deficiency. Plant and Soil. 2006;283:155–162. [Google Scholar]
- Holford JCR. Soil phosphorus: it's measurement, and its uptake by plants. Australian Journal of Soil Research. 1997;35:227–239. [Google Scholar]
- Ikenaga M, Asakawa S, Muraoka Y, Kimura M. Bacterial communities associated with nodal roots of rice plants along with the growth stages: estimation by PCR-DGGE and sequence analyses. Soil Science and Plant Nutrition. 2003;49:591–602. [Google Scholar]
- Impa SM, Johnson-Beebout SE. Mitigating zinc deficiency and achieving high grain Zn in rice through integration of soil chemistry and plant physiology research. Plant and Soil. 2012 in press. http://dx.doi.org/10.1007/s11104-012-1315-3 . [Google Scholar]
- Ishimaru Y, Bashir K, Nishizawa NK. Zn uptake and translocation in rice plants. Rice. 2011;4:21–27. [Google Scholar]
- Ismail AM, Heuer S, Thomson JT, Wissuwa M. Genetic and genomic approaches to develop rice germplasm for problem soils. Plant Molecular Biology. 2007;65:547–570. doi: 10.1007/s11103-007-9215-2. [DOI] [PubMed] [Google Scholar]
- Jia H, Ren H, Gu M, et al. The phosphate transporter gene OsPht1;8 is involved in phosphate homeostasis in rice. Plant Physiology. 2011;156:1164–1175. doi: 10.1104/pp.111.175240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SE, Loeppert RH. Role of organic acids in phosphate mobilization from iron oxide. Soil Science Society of America Journal. 2006;70:222–234. [Google Scholar]
- Johnson-Beebout SE, Lauren JG, Duxbury JM. Immobilization of zinc fertilizer in flooded soils monitored by adapted DTPA soil test. Communications in Soil Science and Plant Analysis. 2009;40:1842–1861. [Google Scholar]
- Kirk GJD. The biogeochemistry of submerged soils. Chichester, UK: John Wiley & Sons; 2004. [Google Scholar]
- Kirk GJD. A model of phosphate solubilization by organic anion excretion from plant roots. European Journal of Soil Science. 1999;50:369–378. [Google Scholar]
- Kirk GJD. Rice root properties for internal aeration and efficient nutrient acquisition in submerged soil. New Phytologist. 2003;159:185–194. doi: 10.1046/j.1469-8137.2003.00793.x. [DOI] [PubMed] [Google Scholar]
- Kirk GJD, Le Van Du. Changes in rice root architecture, porosity, and oxygen and proton release under phosphorus deficiency. New Phytologist. 1997;135:191–200. [Google Scholar]
- Kirk GJD, Saleque MA. Solubilization of phosphate by rice plants growing in reduced soil: prediction of the amount solubilized and the resultant increase in uptake. European Journal of Soil Science. 1995.;46:247–255. [Google Scholar]
- Kirk GJD, Santos EE, Findenegg GR. Phosphate solubilization by organic anion excretion from rice (Oryza sativa L.) growing in aerobic soil. Plant and Soil. 1999a;211:11–18. [Google Scholar]
- Kirk GJD, Santos EE, Santos MB. Phosphate solubilization by organic anion excretion from rice growing in aerobic soil: rates of excretion and decomposition, effects on rhizosphere pH and effects on phosphate solubility and uptake. New Phytologist. 1999b;142:185–200. [Google Scholar]
- Knauth S, Hurek T, Brar D, Reinhold-Hurek B. Influence of different Oryza cultivars on expression of nifH gene pools in roots of rice. Environmental Microbiology. 2005;7:1725–1733. doi: 10.1111/j.1462-2920.2005.00841.x. [DOI] [PubMed] [Google Scholar]
- Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ. Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany. 2006;98:693–713. doi: 10.1093/aob/mcl114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucy M, Reed E, Glick BR. Applications of free-living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek Journal of General and Molecular Microbiology. 2004;86:1–25. doi: 10.1023/B:ANTO.0000024903.10757.6e. [DOI] [PubMed] [Google Scholar]
- Li L, Liu C, Lian X. Gene expression profiles in rice roots under low phosphorus stress. Plant Molecular Biology. 2010;72:423–432. doi: 10.1007/s11103-009-9580-0. [DOI] [PubMed] [Google Scholar]
- Liang Y, Zhu Y-G, Xia Y, Li Z, Ma Y. Iron plaque enhances phosphorus uptake by rice (Oryza sativa) growing under varying phosphorus and iron concentrations. Annals of Applied Biology. 2006;149:305–312. [Google Scholar]
- Liu F, Chang XJ, Ye Y, Xie WB, Wu P, Lian XM. Comprehensive sequence and whole-life-cycle expression profile analysis of the phosphate transporter gene family in rice. Molecular Plant. 2011;4:1105–1122. doi: 10.1093/mp/ssr058. [DOI] [PubMed] [Google Scholar]
- Lynch JP. Roots of the second green revolution. Australian Journal of Botany. 2007;55:493–512. [Google Scholar]
- Ma Q, Rengel Z, Rose TJ. The effectiveness of deep placement of fertilizers is determined by crop species and edaphic conditions in Mediterranean-type environments: a review. Australian Journal of Soil Research. 2009;47:19–32. [Google Scholar]
- Maiti D, Variar M, Singh RK. Optimizing tillage schedule for maintaining activity of the arbuscular mycorrhizal fungal population in rainfed upland rice (Oryza sativa L.) agroecosystem. Mycorrhiza. 2011a;3:167–171. doi: 10.1007/s00572-010-0324-4. [DOI] [PubMed] [Google Scholar]
- Maiti D, Toppo NN, Variar M. Integration of crop rotation and arbuscular mycorrhiza (AM) inoculums application for enhancing AM activity to improve phosphorus nutrition and yield of upland rice (Oryza sativa L.) Mycorrhiza. 2011b;8:659–667. doi: 10.1007/s00572-011-0376-0. [DOI] [PubMed] [Google Scholar]
- Malusa E, Laurenti E, Juszczuk I, Ferrari RP, Rychter AM. Free radical production production in roots of Phaseolus vulgaris subjected to phosphate deficiency stress. Plant Physiology and Biochemistry. 2002;40:963–967. [Google Scholar]
- Mei XQ, Ye ZH, Wong MH. The relationship of root porosity and radial oxygen loss on arsenic tolerance and uptake in rice grains and straw. Environmental Pollution. 2009;157:2550–2557. doi: 10.1016/j.envpol.2009.02.037. [DOI] [PubMed] [Google Scholar]
- Morcuende R, Bari R, Gibon Y, et al. Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant, Cell & Environment. 2007;30:85–112. doi: 10.1111/j.1365-3040.2006.01608.x. [DOI] [PubMed] [Google Scholar]
- Muchhal US, Raghothama KG. Transcriptional regulation of plant phosphate transporters. Proceedings of the National Academy of Sciences of the USA. 1999;96:5868–5872. doi: 10.1073/pnas.96.10.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozoye T, Nagasaka S, Kobayashi T, et al. Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. Journal of Biological Chemistry. 2011;286:5446–5454. doi: 10.1074/jbc.M110.180026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oburger E, Kirk GJD, Wenzel WW, Puschenreiter M, Jones DL. Interactive effects of organic acids in the rhizosphere. Soil Biology and Biochemistry. 2009;41:449–457. [Google Scholar]
- de Oliveira CA, Marriel IE, Gomes EA, de Paula Lana UG, Scotti MR, Alves VMC. Bacterial diversity in the rhizosphere of maize genotypes contrasting for phosphorus use efficiency. Pesquisa Agropecuária Brasileira. 2009a;44:1473–1482. [Google Scholar]
- de Oliveira CA, Sa NMH, Gomes EA, et al. Assessment of the mycorrhizal community in the rhizosphere of maize (Zea mays L.) genotypes contrasting for phosphorus efficiency in the acid savannas of Brazil using denaturing gradient gel electrophoresis (DGGE) Applied Soil Ecology. 2009b;41:249–258. [Google Scholar]
- Pariasca-Tanaka J, Satoh K, Rose T, Mauleon R, Wissuwa M. Stress response versus stress tolerance: a transcriptome analysis of two rice lines contrasting in tolerance to phosphorus deficiency. Rice. 2009;2:167–185. [Google Scholar]
- Paszkowski U, Kroken S, Roux C, Briggs SP. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proceedings of the National Academy of Sciences of the USA. 2002;99:13324–13329. doi: 10.1073/pnas.202474599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashnyk M, Roose T, Jones DL, Kirk GJD. Enhanced zinc uptake by rice through phytosiderophore secretion: a modelling study. Plant, Cell & Environment. 2011;34:2038–2046. doi: 10.1111/j.1365-3040.2011.02401.x. [DOI] [PubMed] [Google Scholar]
- Quijano-Guerta C, Kirk GJD, Portugal AM, Bartolome VI, McLaren GC. Tolerance of rice germplasm to zinc deficiency. Field Crops Research. 2002;76:123–130. [Google Scholar]
- Rae AL, Jarmey JM, Mudge SR, Smith FW. Over-expression of a high-affinity phosphate transporter in transgenic barley plants does not enhance phosphate uptake rates. Functional Plant Biology. 2004;31:141–148. doi: 10.1071/FP03159. [DOI] [PubMed] [Google Scholar]
- Raghothama KG. Phosphate acquisition. Annual Review of Plant Physiology. 1999;50:665–693. doi: 10.1146/annurev.arplant.50.1.665. [DOI] [PubMed] [Google Scholar]
- Rengel Z. Root exudation and microflora populations in the rhizosphere of crop genotypes differing in tolerance to micronutrient deficiency. Plant and Soil. 1997;196:255–260. [Google Scholar]
- Richardson AE, Simpson RJ. Soil microorganisms mediating phosphorus availability. Plant Physiology. 2011;156:989–996. doi: 10.1104/pp.111.175448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AE, Hocking PJ, Simpson RJ, George TS. Plant mechanisms to optimise access to soil phosphorus. Crop and Pasture Science. 2009;60:124–143. [Google Scholar]
- Richardson AE, Lynch JP, Ryan PR, et al. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant and Soil. 2011;349:121–156. [Google Scholar]
- Rose MT, Pariasca-Tanaka J, Rose TJ, Wissuwa M. Bicarbonate tolerance of Zn-efficient rice genotypes is not related to organic acid exudation, but to reduced solute leakage from roots. Functional Plant Biology. 2011a;38:493–504. doi: 10.1071/FP11008. [DOI] [PubMed] [Google Scholar]
- Rose MT, Rose TJ, Pariasca-Tanaka J, et al. Root metabolic response of rice (Oryza sativa L.) genotypes with contrasting tolerance to zinc deficiency and bicarbonate excess. Planta. 2012 doi: 10.1007/s00425-012-1648-4. in press. http://dx.doi.org/10.1007/s00425-012-1648-4 . [DOI] [PubMed] [Google Scholar]
- Rose TJ, Wissuwa M. Advances in agronomy. Vol. 116. New York, NY: American Society of Agronomy and Academic Press; 2012. Rethinking internal phosphorus utilization efficiency (PUE): a new approach is needed to improve PUE in grain crops. In: Sparks DL; pp. 185–217. [Google Scholar]
- Rose TJ, Hardiputra B, Rengel Z. Wheat, canola and grain legume access to soil phosphorus fractions differs in soils with contrasting phosphorus dynamics. Plant and Soil. 2010;326:159–170. [Google Scholar]
- Rose TJ, Rose MT, Pariasca-Tanaka J, Heuer S, Wissuwa M. The frustration with utilization: why have improvements in internal phosphorus utilization efficiency in crops remained so elusive? Frontiers in Plant Nutrition. 2011b;2:1–5. doi: 10.3389/fpls.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouached H, Stefanovic A, Secco D, et al. Uncoupling phosphate deficiency from its major effects on growth and transcriptome via PHO1 expression in Arabidopsis. The Plant Journal. 2011;65:557–570. doi: 10.1111/j.1365-313X.2010.04442.x. [DOI] [PubMed] [Google Scholar]
- Schefe CR, Watt M, Slattery WJ, Mele PM. Organic anions in the rhizosphere of Al-tolerant and Al-sensitive wheat lines grown in an acid soil in controlled and field conditions. Australian Journal of Soil Research. 2008;46:257–264. [Google Scholar]
- Seyfferth AL, Webb SM, Andrews JC, Fendorf S. Arsenic localization, speciation, and co-occurrence with iron on rice (Oryza sativa L.) roots having variable Fe coatings. Environmental Science and Technology. 2010;44:8108–8113. doi: 10.1021/es101139z. [DOI] [PubMed] [Google Scholar]
- Sharpley AN, McDowell RW, Kleinman PJA. Phosphorus loss from land to water: integrating agricultural and environmental management. Plant and Soil. 2001;237:287–307. [Google Scholar]
- Smith SE, Smith FA. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annual Review of Plant Biology. 2011;62:227–250. doi: 10.1146/annurev-arplant-042110-103846. [DOI] [PubMed] [Google Scholar]
- Solaiman MZ, Hirata H. Responses of directly seeded wetland rice to arbuscular mycorrhizal fungi inoculation. Journal of Plant Nutrition. 1997;20:1479–1487. [Google Scholar]
- Solaiman MZ, Hirata H. Glomus-wetland rice mycorrhizas influenced by nursery inoculation techniques under high fertility soil conditions. Biology and Fertility of Soils. 1998;27:92–96. [Google Scholar]
- Sǿrensen J, Nicolaisen MH, Ron E, Simonet P. Molecular tools in rhizosphere microbiology: from single-cell to whole-community analysis. Plant and Soil. 2009;321:483–512. [Google Scholar]
- Steen I. Phosphorus availability in the 21st Century: management of a nonrenewable resource. Phosphorus and Potassium. 1998;217:25–31. [Google Scholar]
- Suzuki K, Okazaki K, Tawaraya K, Osaki M, Shinano T. Gas chromatography–mass spectrometry associated global analysis of rice root exudates under aseptical conditions. Soil Science and Plant Nutrition. 2009;55:505–513. [Google Scholar]
- Suzuki M, Tsukamoto T, Inoue H, et al. Deoxymugineic acid increases Zn translocation in Zn-deficient rice plants. Plant Molecular Biology. 2008;66:609–617. doi: 10.1007/s11103-008-9292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z, Hurek T, Reinhold-Hurek B. Effect of N-fertilization, plant genotype and environmental conditions on nifH gene pools in roots of rice. Environmental Microbiology. 2003;5:1009–1015. doi: 10.1046/j.1462-2920.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- Tawaraya K, Horie R, Shinano T, Wagatsuma T, Saito K, Oikawa A. Metabolite profiling of rice root exudate under phosphorus deficiency. Proceedings of the International Plant Nutrition Colloquium XVI, Department of Plant Sciences, UC Davis, UC Davis. 2009 http://escholarship.org/uc/item/5n23m80d . [Google Scholar]
- Tariq M, Hameed S, Malik KA, Hafeez FY. Plant root associated bacteria for zinc mobilization in rice. Pakistan Journal of Botany. 2007;39:245–253. [Google Scholar]
- Turner FT, Gillam JW. Increased P diffusion as an explanation of increased P availability in flooded soils. Plant and Soil. 1976;45:365–377. [Google Scholar]
- Uga Y, Okuno K, Yano M. Dro1, a major QTL involved in deep rooting of rice under upland field conditions. Journal of Experimental Botany. 2011;62:2485–2494. doi: 10.1093/jxb/erq429. [DOI] [PubMed] [Google Scholar]
- Uga Y, Hanzawa Y, Nagai S, Sasaki K, Yano M, Sato T. Identification of qSOR1, a major rice QTL involved in soil-surface rooting in paddy fields. Theoretical and Applied Genetics. 2012;124:75–86. doi: 10.1007/s00122-011-1688-3. [DOI] [PubMed] [Google Scholar]
- Vessey JK. Plant growth promoting rhizobacteria as bio-fertilizers. Plant and Soil. 2003;255:571–586. [Google Scholar]
- Vu DT, Tang C, Armstrong RD. Changes and availability of P fractions following 65 years of P application in a calcareous soil in a Mediterranean region. Plant and Soil. 2008;304:21–33. [Google Scholar]
- Widodo , Broadley MR, Rose TJ, et al. Response to zinc deficiency of two rice lines with contrasting tolerance is determined by root growth maintenance and organic acid exudation rates, and not by zinc-transporter activity. New Phytologist. 2010;186:400–414. doi: 10.1111/j.1469-8137.2009.03177.x. [DOI] [PubMed] [Google Scholar]
- Wissuwa M. How do plants achieve tolerance to phosphorus deficiency? Small causes with big effects. Plant Physiology. 2003;133:1947–1958. doi: 10.1104/pp.103.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissuwa M, Ae N. Genotypic variation for tolerance to phosphorus deficiency in rice and the potential for its exploitation in rice improvement. Plant Breeding. 2001a;120:43–48. [Google Scholar]
- Wissuwa M, Ae N. Further characterization of two QTLs that increase phosphorus uptake of rice (Oryza sativa L.) under phosphorus deficiency. Plant and Soil. 2001b;237:275–286. [Google Scholar]
- Wissuwa M, Wegner J, Ae N, Yano M. Substitution mapping of Pup1: a major QTL increasing phosphorus uptake of rice from a phosphorus-deficient soil. Theoretical and Applied Genetics. 2002;105:890–897. doi: 10.1007/s00122-002-1051-9. [DOI] [PubMed] [Google Scholar]
- Wissuwa M, Gamat G, Ismail A. Is root growth under phosphorus deficiency affected by source or sink limitations? Journal of Experimental Botany. 2005;56:1943–1950. doi: 10.1093/jxb/eri189. [DOI] [PubMed] [Google Scholar]
- Wissuwa M, Ismail AM, Yanagihara S. Effects of zinc deficiency on rice growth and genetic factors contributing to tolerance. Plant Physiology. 2006;142:731–741. doi: 10.1104/pp.106.085225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissuwa M, Mazzola M, Picard C. Novel approaches in plant breeding for rhizosphere-related traits. Plant and Soil. 2009;321:409–430. [Google Scholar]
- Wu Z, Ren H, McGrath SP, Wu P, Zhao F-J. Investigating the contribution of the phosphate transport pathway to arsenic accumulation in rice. Plant Physiology. 2011;157:498–508. doi: 10.1104/pp.111.178921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Xu X, Fukao T, Canlas P, et al. Sub1A is an ethylene-responsive-factor-like gene that confers submergence tolerance to rice. Nature. 2006;442:705–708. doi: 10.1038/nature04920. [DOI] [PubMed] [Google Scholar]
- Zhang XK, Zhang FS, Mao DR. Effect of Fe plaque outside roots on nutrient uptake by rice (Oryza sativa L.): zinc uptake. Plant and Soil. 1998;202:33–39. [Google Scholar]
- Zhang XK, Zhang FS, Mao DR. Effect of iron plaque outside roots on nutrient uptake by rice (Oryza sativa L.): phosphorus uptake. Plant and Soil. 1999;209:187–192. [Google Scholar]