Abstract
Background and Aims
The maize lrt1 (lateral rootless1) mutant is impaired in its development of lateral roots during early post-embryonic development. The aim of this study was to characterize, in detail, the influences that the mutation exerts on lateral root initiation and the subsequent developments, as well as to describe the behaviour of the entire plant under variable environmental conditions.
Methods
Mutant lrt1 plants were cultivated under different conditions of hydroponics, and in between sheets of moist paper. Cleared whole mounts and anatomical sections were used in combination with both selected staining procedures and histochemical tests to follow root development. Root surface permeability tests and the biochemical quantification of lignin were performed to complement the structural data.
Key Results
The data presented suggest a redefinition of lrt1 function in lateral roots as a promoter of later development; however, neither the complete absence of lateral roots nor the frequency of their initiation is linked to lrt1 function. The developmental effects of lrt1 are under strong environmental influences. Mutant primordia are affected in structure, growth and emergence; and the majority of primordia terminate their growth during this last step, or shortly thereafter. The lateral roots are impaired in the maintenance of the root apical meristem. The primary root shows disturbances in the organization of both epidermal and subepidermal layers. The lrt1-related cell-wall modifications include: lignification in peripheral layers, the deposition of polyphenolic substances and a higher activity of peroxidase.
Conclusions
The present study provides novel insights into the function of the lrt1 gene in root system development. The lrt1 gene participates in the spatial distribution of initiation, but not in its frequency. Later, the development of lateral roots is strongly affected. The effect of the lrt1 mutation is not as obvious in the primary root, with no influences observed on the root apical meristem structure and maintenance; however, development of the epidermis and cortex are impaired.
Keywords: Zea mays, lrt1, lateral root, lateral root emergence, root apical meristem, lignin, peroxidase
INTRODUCTION
Lateral roots (LRs) typically form the major portion of the root systems of vascular plants. Root system architecture, which is shaped by root branching, is one of the key agronomic parameters determining the uptake of water, nutrients and other substances, as well as the eco-physiological relationships with the rhizosphere.
LRs are mostly formed postembryonally in the subapical zone of the parent root (Esau, 1965). Their development has been studied in detail in the Arabidopsis thaliana dicot model, primarily due to its simple and experimentally accessible root system. Initiation of its LRs takes place in the protoxylem sector of the pericycle, and is normally divided into eight steps, including formation of founder cells, anticlinal and periclinal divisions, as well as the activation of a new apical meristem during emergence from the maternal root tissues (Malamy and Benfey, 1997). LR initiation in more complex (and agronomically important) model plants such as the monocot cereals maize (Zea mays) and rice (Oryza sativa) differ to some extent. In maize, LR initiation takes place opposite from the phloem poles of the polyarch stele (Bell and McCully, 1970; Jansen et al., 2012). Most of the young LRs are formed from pericycle cells, except for the temporary early root cap of endodermal origin, which are later replaced by the activity of the root apical meristem initials (Clowes, 1978). The heterogeneity of the cereal root system and its development is reflected in the specific root-type regulatory mechanisms (Majer and Hochholdinger, 2011).
Intrinsic mechanisms provide a baseline of the root system's architectural set-up. Auxin plays a pivotal role in root development by interacting with other plant growth regulators, as well as other regulatory factors (Tung et al., 1996; Zimmermann et al., 2010; Kapulnik et al., 2011; Smith and De Smet, 2012). External factors allow for a modification of the intrinsic programme within the limits of developmental plasticity (Malamy, 2005), in order to adjust to and cope with the conditions of the environment.
There are a plethora of root development mutants in A. thaliana, yet only a limited number of root mutants are currently available for other plant species. Study of the interspecific differences in root development are of particular value. The tomato (Lycopersicon esculentum) mutant dgt (diageotropica) does not generate LRs as a result of changes in auxin signalling (Muday et al., 1995; Lavy et al., 2012). Similarly, rice (Oryza sativa) mutants without LRs were described as lrt1 (lateral rootless 1; Chhun et al., 2003b), lrt2 (lateral rootless 2; Wang et al., 2006) and RM109 (Rice Mutant 109; Hao and Masahiko, 1999). Moreover, rice mutants with reduced numbers of LRs include arm1, arm2 (auxin-resistant mutant 1 and 2; Chhun et al., 2003a), and crl1 (crown rootless 1; Inukai et al., 2005). Lastly, several mutants with defects in their LR development have been identified in maize (Zea mays), including the mutants slr1, slr2 (short lateral roots 1 and 2; Hochholdinger et al., 2001), rum1 (rootless with undetectable meristems 1; Woll et al., 2005) and lrt1 (lateral rootless 1; Hochholdinger and Feix, 1998).
The monogenic and recessive mutant lrt1 was isolated from a segregating F2-generation of an EMS (ethyl methane sulfonate) mutagenized population (Hochholdinger and Feix, 1998) of the B73 inbred line, due to its conspicuous phenotype. The lrt1 mutant is deficient in LR formation during its early postembryonic development (Hochholdinger and Feix, 1998). Partial recovery was reported in the presence of arbuscular mycorrhiza, and was further pronounced in the presence of a high phosphate concentration (Paszkowski and Boller, 2002). However, such LRs were short, highly branched, and with a distribution along the primary root far from the normal acropetal sequence. The mutant phenotype does not seem to be directly related to auxin signalling; it was not reversed by exogenous application of auxin (Hochholdinger and Feix, 1998), nor were any disturbances in the PIN1 efflux transporter localization observed (Schlicht et al., 2006). Based on an analysis of lrt1, the role of the ZmGSL (Gibberellic Acid Stimulated-Like) gene family, during formation of LR primordium, was revealed (Zimmermann et al., 2010).
Detailed histological studies of this mutant, presented here, provide further insights into the function of the lrt1 gene, which is used as a reference lateral rootless material (Hochholdinger et al., 2004a; Park et al., 2004; Schlicht et al., 2006; Zimmermann et al., 2010) for studies of maize root development.
MATERIAL AND METHODS
Plant materials and growth conditions
Seeds of a segregating Zea mays lrt1 and wild-type (B73 genotype) were imbibed for 4 d at 4 °C, and then germinated on moist filter paper in the dark at 27 °C for 4 days. Seedlings with approx. 2 cm primary root were mounted into 5-litre containers in the growth chamber (16/8-h photoperiod; irradiation 435 W m−2 photosynthetically active radiation; 22/18 °C day/night; relative humidity approx. 50 %). Quarter-strength Hoagland 3 solution (Hoagland and Amon, 1950), supplemented with microelements [120 nm H3BO3, 360 nm MnCl2.4H2O, 5 nm ZnSO4.7H2O, 0·3 nm (NH4)6Mo7O24, 0·9 nm CuSO4.5H2O (Arnon, 1938) and 10 µm Fe3+ citrate (pH 5·3–5·5)], was used and changed weekly. The plants were either cultivated between moist sheets of filter paper (Lenochová et al., 2009) or cultivated using hydroponics, which was either constantly aerated (dissolved oxygen saturation ≥60 %) or stagnant (in which the oxygen content during 3 days gradually decreased to 5–10 % of saturation). The plants were harvested 16 days post germination, scanned, and the shoot and root lengths were measured. Long-term hydroponics was carried out in a greenhouse in 80-litre reservoirs, with internal circulation of the solution (dissolved oxygen saturation ≥75 %) for approx. 130 days until flowering. The composition of the nutrient solution was identical to that of the short-term cultivations. It was changed every other week, with the microelements and iron supplemented weekly.
Anatomy and histochemistry
Samples from selected positions of the primary roots from 16-d-old plants were either fixed in 50 % FAA for the permanent sections, or in 4 % formaldehyde in phosphate buffer (0·05 m, pH 6·8) for the free-hand sections. Selected segments of the primary root were embedded into histoplast and longitudinal as well as transversal sections (10 µm) were cut; they were then stained with Safranine O and Fast Green FCF (Johansen, 1940). Quantitative image analyses were performed using NIS Elements software (Laboratory Imaging; http://www.nis-elements.com/).
Free-hand transverse sections (approx. 150 µm) were stained in Sudan Red 7B (0·01 %, w/v) (Brundrett et al., 1991), HCl-phloroglucinol (Jensen, 1962), and berberine hemisulfate (0·1 % aq.; UV Olympus U-MWU filter block) (Brundrett et al., 1988); and counterstained with Crystal Violet (0·05 % aq., 10 min, for quench autofluorescence; Grubler). Unstained control sections were surveyed in bright field or UV-excited autofluorescence.
Peroxidase activity was detected histochemically on free-hand sections after 2 h of formaldehyde fixation, as described above. Reaction mixtures containing 1 mL acetate buffer (0·1 m, pH 5) with a 282-μL mixture of diaminobenzidine (0·78 mg mL−1), NiCl2 (4 mg mL−1) and H2O2 (0·03 mg mL−1) was applied to sections for 1 h at 35 °C in the dark. H2O2 was omitted from control sections, and peroxidise inhibition with phenylhydrazine (5 min; 0·1 % in phosphate-buffered saline) was alternatively used (Pearse, 1968).
The permeability of intact root surfaces was probed for 1 h with periodic acid 0·02 % aq. solution, and penetration of the solution from the surroundings was localized on free-hand sections, as previously described (Soukup et al., 2007). Control sections were processed without periodic acid oxidation.
Sections were observed with an Olympus BX51 microscope (Olympus Corp., Tokyo, Japan) (differential interference contrast, epifluorescence), and documented with an Apogee U4000 digital camera (Apogee Imaging Systems, Inc., Roseville, CA, USA). NaI solution (Dubrovsky et al., 2009) cleared the root tips stained with propidium iodide (5 µg mL−1; MP) and Hoechst 33258 (2 µg mL−1; Sigma). These were then observed in a confocal laser scanning microscope (TCS SP2; Leica, Mannheim, Germany).
Segments of primary roots (length 1 cm) were fixed for 24 h in 2·5 % glutaraldehyde in cacodylate buffer (0·1 m, pH 7·2), and then processed for scanning electron microscopy (JSM-6380LV; JEOL, Tokyo, Japan) as follows: dehydration through a gradual ethanol and acetone series, transfer to tertiary butanol, vacuum drying, followed by gold coating (Sputter Coater SCD 050, Bal-Tec).
Analysis of developmental stages and quantification of primordia/LRs
The developmental stages of LR primordia were quantified from 1-cm-long root segments, using permanent longitudinal serial sections. The frequency of LR initiation was expressed as a LR initiation index ILRI = 100 × d × l, where d is the density of LR initiation events (number mm−1) and l is the average fully elongated cortical cell length (mm) (Dubrovsky et al., 2009).
Quantification of lignin
Samples of selected root parts of plants grown for 16 and 130 d in a hydroponic system were dried at 60 °C and homogenized. The cell walls were purified (Selvendran, 1975) and incubated with thioglycolic acid (Bruce and West, 1989). The LTGAs (lignin thioglycolic acid derivates) were successively extracted from the cell walls and measured spectrophotometrically (Helios α; Unicam, Cambridge, UK) at 280 nm. The results were expressed as absorbance per dry weight of purified cell walls (Lange et al., 1995).
Statistical analyses
A two-sample t-test, and in the case of data without a normal distribution the Mann–Whitney/Wilcoxon or Kolmogorov–Smirnov non-parametric tests were applied (NCSS 2001; Jerry Hintze, Kaysville, UT, USA). For testing of differences in the proportion of particular developmental stages of primordium/LRs within the tested position of an entire root of wild-type and lrt1 plants, Hotelling's two-sample t-test was used (Hotelling, 1931). The Bonferroni–Holm method was used to maintain the P-value for non-independent samples (Holm, 1979). Error bars within the plots indicate the standard errors (s.e.) of the mean.
RESULTS
The lrt1 affects overall shoot and root sizes
Overall, mutant lrt1 plants were more delicate than the wild-type phenotype, displaying fewer leaves, as well as shorter shoots and roots. Shoot and root elongation was affected according to the mode of cultivation as quantified for 16-d-old plants grown in moist paper, or alternatively, either in an aerated or non-aerated hydroponic culture (Table 1). The longest primary roots were obtained under aerated hydroponics. Wild-type primary roots, on average, were 40 % longer than the lrt1 mutants under such conditions. Non-aerated hydroponics led to shorter primary roots; the wild-type were only 11 % longer than in the lrt1; those grown in moist paper sheets were 89 % longer than their lrt1 siblings. In contrast, shoots grew best under non-aerated hydroponic conditions; they grew worst in moist paper. On average, wild-type shoots were between 43 and 57 % longer than the lrt1 shoots at this stage of development.
Table 1.
Shoot and root length (mm, mean ± s.e.) of 16-d-old wild-type and lrt1 seedlings under different cultivation conditions
| Moist paper (n = 5) |
Non-aerated hydroponics (n = 8) |
Aerated hydroponics (n = 8) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shoot |
Root |
Shoot |
Root |
Shoot |
Root |
||||||
| WT | lrt1 | WT | lrt1 | WT | lrt1 | WT | lrt1 | WT | lrt1 | WT | lrt1 |
| 106·0 ± 2·62 | 69·5 ± 2·7 | 269·6 ± 10·9 | 142·7 ± 4·2 | 293·6 ± 13·9 | 187·5 ± 6·3 | 180·0 ± 3·1 | 162·3 ± 7·0 | 231·6 ± 3·7 | 162·5 ± 2·8 | 355·6 ± 9·9 | 253·1 ± 6·1 |
| ** | ** | ** | ** | ** | ** | * | * | ** | ** | ** | ** |
Asterisks indicate level of significance from two-sample t-test: *P < 0·05, ** P < 0·01.
The lrt1 affects root system architecture in an environmentally sensitive manner
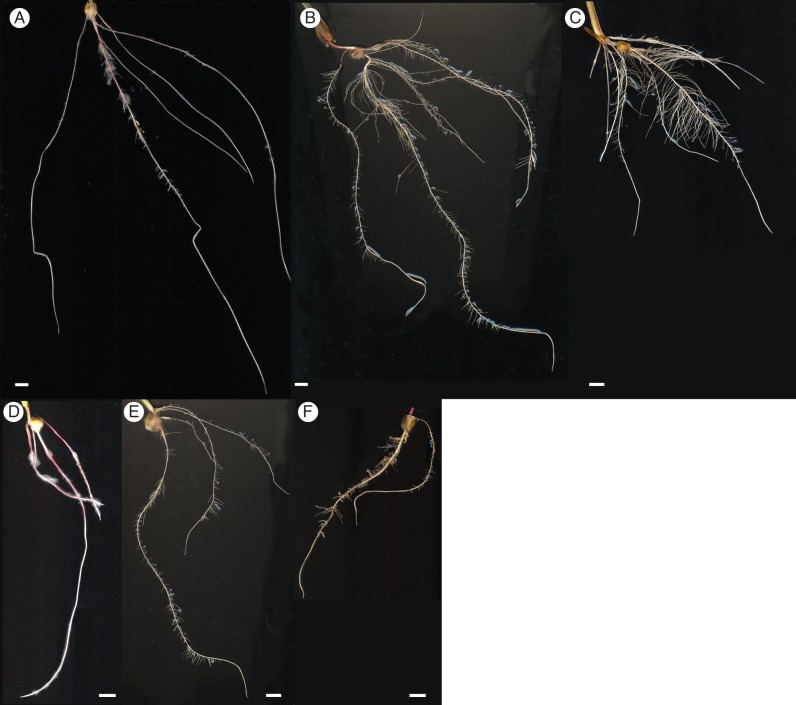
LR formation was analysed in the 16-d-old plants under the cultivation conditions described above. Growth in moist paper had the strongest inhibitory effect on the branching of both wild-type and mutant roots (Fig. 1A, D). While LRs were delayed, but still formed in the wild-type, no LRs were present in the lrt1 mutant on primary or seminal roots (Fig. 1D). Both the aerated (Fig. 1B, E) and the non-aerated (Fig. 1C, F) hydroponic cultured plants formed frequent LRs on all root types of the wild-type (Fig. 1B, C), as well as the lrt1 mutant (Fig. 1E, F) seedlings. Overall, the root systems from hydroponics were more complex than from paper cultivation, with the wild-type plants developing longer LRs, and more branched seminal roots. Mutant roots in non-aerated hydroponics (Fig. 1F) were brownish, with traces of decay.
Fig. 1.
Morphology of wild-type and lrt1 root systems. Wild type (A–C) and mutant lrt1 (D–F) plants grown under various growth conditions for 16 days. Compared with wild-type plants (A), no LR emergence was observed in lrt1 plants (D) cultivated in moist paper. In contrast, LRP emerged in aerated hydroponics (wild-type, B; lrt1, E) and non-aerated hydroponics (wild-type, C; lrt1, F), respectively. In non-aerated hydroponics, mutants (F) displayed signs of decay, illustrated by brownish roots. The majority of LRs in lrt1 mutants (E, F) terminated their growth during or shortly after emergence. Scale bars = 10 mm.
A lower root system complexity, with less branching and very short LRs, was also observed for lrt1 in long-term cultivation (Supplementary Data Fig. S1).
The lrt1 largely affects the latter stages of lateral root development
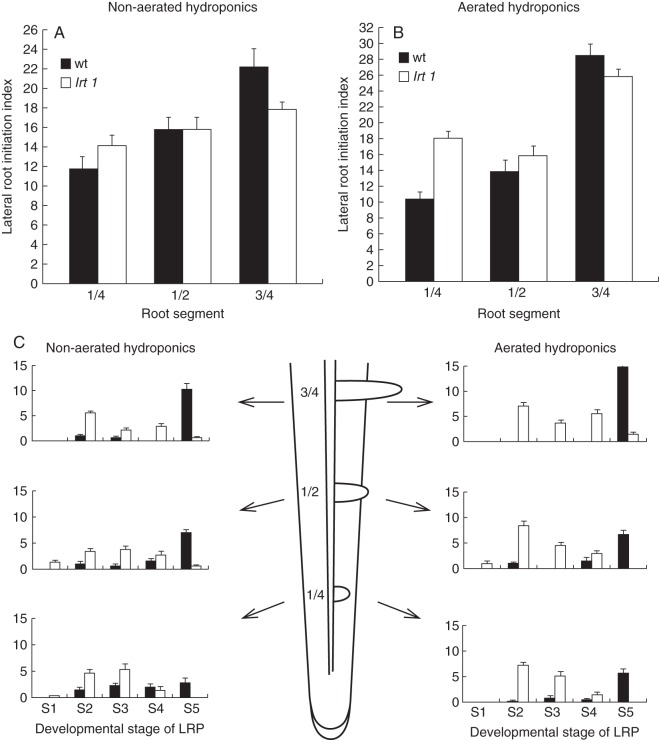
Bulges of lateral root primordia (LRP) were observed on the surface of lrt1 primary roots 6 d after germination in both hydroponic cultivations, and about 2 d later in those germinated in moist paper. LR density was calculated for 16-d-old plants grown in hydroponics, and expressed as an LR initiation index (Fig. 2A, B). There was only a minor effect of lrt1 on the frequency of LR initiation. LRP were initiated at comparable or even higher rates (in aerated hydroponics) in lrt1 mutants (two-sample t-test, P < 0·01, n = 8).
Fig. 2.
Quantification of LRP initiation and LR growth (A, B) and analysis of developmental stages of LRP (C). Non-aerated hydroponics (A), aerated hydroponics (B). Mutants initiate comparable numbers of LRs as the wild-type, and in some cases the LR initiation index was even higher in aerated hydroponics (two-sample t-test, P < 0·01). (C) Number of individual developmental stages of LRP (S1–S5 as specified in the text). Fraction of individual developmental stages of LRP changes along the root: from the first quarter of its length, the middle portion and the third quarter of the length from the tip of the primary root. A significantly lower percentage of LRP emergence (S4–S5) was observed in lrt1 roots, regardless of the cultivation conditions. Proportions of the developmental stages along the root, as well as within the tested root position, were statistically different for lrt1 and the wild-type. The differences were statistically significant (Hotelling's two-sample t-test, P < 0·05). Values shown are mean numbers of individual developmental stages of LRP within the segment and s.e. (n = 8).
The complete developmental process of LRP has been divided into five stages for simplified quantification (Fig. 2C). The first stage included the first anticlinal and periclinal divisions (maximum of two cell layers) in the pericycle. In the second stage, the LRP grew to half way through the cortex of the primary root. During the third stage, the LRP reached the peripheral layers of the primary root cortex. In the fourth stage, the LRs emerged from the maternal root. Finally, the last stage was reached when the LRs emerged from the primary root by more than 500 µm. We tested fractions of particular developmental stages of primordium/LRs for specified root positions or for the entire root. In both cases, lrt1-related differences were detected (P < 0·05; Hotelling's two-sample t-test, n = 8). Defects in the development of later (post initiation) stages of LRP were pronounced in an increased proportion of the early (the second and the third) stages of LRP in lrt1. The early stages were present at a high frequency in lrt1 primary roots, independent of the culture conditions, even in the basal portions among fully emerged LRs. This situation was not observed in the wild-type. Emergence of mutant LRs began significantly further away from the root tip in lrt1 roots. In aerated hydroponics, the part of the primary root without emerged LRs was twice as long (1192·3 ± 7·6) as the wild-type (584·6 ±2·0). In non-aerated hydroponics, it was extended by 76 % (lrt1: 356·0 ±2·4, wild-type: 202·1 ±4·6); in moist paper it was extended by 36 % (lrt1: 949·2 ± 5·7, wild-type: 697·3 ± 10·0) (two-sample t-test, P < 0·01, n = 5–8, means ± s.e.). A high percentage of lrt1 LRPs do not emerge from the primary root (51 % fewer in the lrt1 roots from non-aerated hydroponics, 69 % fewer in aerated hydroponics) (Fig. 2C).
Anatomy and growth of LRP are defective in lrt1
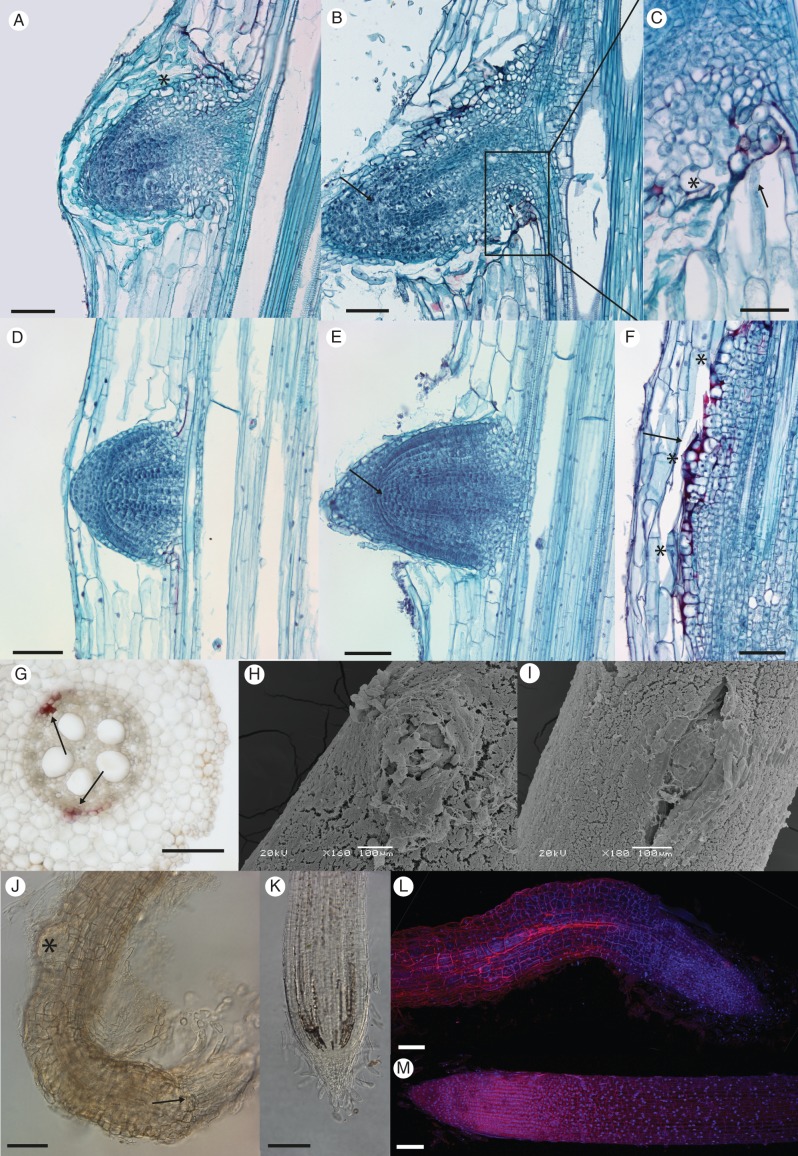
The structure of lrt1 LRP was severely affected in hydroponics (Fig. 3; Supplementary Data Fig. S2). LRP of the early stages (S1–S3) were often noticeably wider and not well delimited (Fig. 3A) when comparing lrt1 with the wild-type (Fig. 3D), often resulting in a mass of dividing cells of the pericycle, with a spatial arrangement deviating from standard LRP development (Supplementary Data Fig. S2). The surface layers of lrt1 LRP of later stages often contained anomalously expanded and highly vacuolated cells (Fig. 3A, C). Anomalous volume growth and the loose arrangement of epidermal cells was far more obvious in the emerged LRs of lrt1 (Fig. 3B, C, J, L). The typical structure of LRP, and later closed apical meristem structure with separated tiers of initials (and their derivatives), was lost in lrt1 seedlings before emergence, or shortly thereafter (Fig. 3B, compare with wild-type in Fig. 3E; see also Supplementary Data Fig. S2). Development of the lrt1 LRP was often associated with ectopic lignifications. Such an anomalous modification of the cell walls was often observed on the base of older LRP (Fig. 3C), as well as in the vicinity of fused and unconventionally spaced bulges of dividing cells, resembling LRP (Fig. 3F). Anomalous lignification was also detected in the pericycle of primary roots between protoxylem poles, where initiation of LRs normally takes place (Fig. 3G).
Fig. 3.
Changes in anatomy and emergence of lrt1 LRP. Plants were cultivated for 16 d in non-aerated hydroponics. LRP of the lrt1 mutant display an altered structure before (A) and after (B) emergence compared with the wild-type (D, E). Anomalously expanded highly vacuolated cells were observed on the surface and on the base of lrt1 LRP (A, C, asterisks). A loose arrangement of epidermal cells was obvious in the emerged lrt1 LRs (B). The structure of root apical meristem of LRP, and later in the apex of LRs with separation of root cap and tiers of initials and their derivatives is lost in lrt1 (B, arrow) compared with the wild-type (E, arrow). Lignifications (red) were often detected in lrt1 on the base of emerged LRP (C, arrow), around the malformed LRP (F, arrow; asterisks mark individual LRP), and in the pericycle of the primary root where initiation normally takes place (G, arrows). Emergence of LRs in lrt1 (H) and separation of the surface cell layers is modified; see (I) for the wild-type. The lrt1 mutant (J) shows irregular divisions in the surface area of LR tips and disorganization in the root apical meristem (J, arrow). Second-order LRP develop very close to first-order LR tips in lrt1 (J, asterisk), which often show strong curvature. Arrangement and anomalous cell volume growth is obvious in the epidermis of LRs (J, L, for lrt1; K, M, for the wild-type ). (A–F) Longitudinal permanent sections stained with Safranine and Fast Green. The sections were prepared from the first quarter of the length from the tip of the primary root. (G) Free-hand section from the middle of the primary root, HCl-phloroglucinol. (H, I) Scanning electron micrographs showing samples from the first quarter from the tip of the primary root. (J–M) Roots were cleared with NaI and stained with propidium iodide and Hoechst 33258. (J, K) Differential interference contarst microscopy; (L, M) confocal laser scanning microscopy, maximal projection. Scale bars = 100 µm, except (C) = 50 µm.
The progress of LR emergence in lrt1 (Fig. 3H) was strongly affected compared with the wild-type (Fig. 3I). The tissues of the penetration site above the LRP did not separate normally, and emergence was probably impeded in lrt1. LRs that emerge from an lrt1 primary root continue growth for a short period of time, and then terminate their growth very soon after emergence (usually no longer than 0·5 mm). The structure of the LR apical meristem is extremely disturbed (Fig. 3J, L); tiers of initials are not distinguishable as in the wild-type (Fig. 3K, M). Second-order LRP start to develop very close to the first-order LR tip in mutant root systems (Fig. 3J); however, this does not take place in the wild-type. The infrequently long LRs of lrt1 (longer than 0·5 mm) are thicker, with randomly curved tips, as well as having anomalous arrangements and swelling of the epidermal cell layers.
The lrt1 takes part in primary root development
The effect of the mutation was not as obvious in the primary root as it was in LRs. The roots of lrt1 were thicker, primarily due to lateral expansion of the cortical cells (Supplementary Data Table S1).
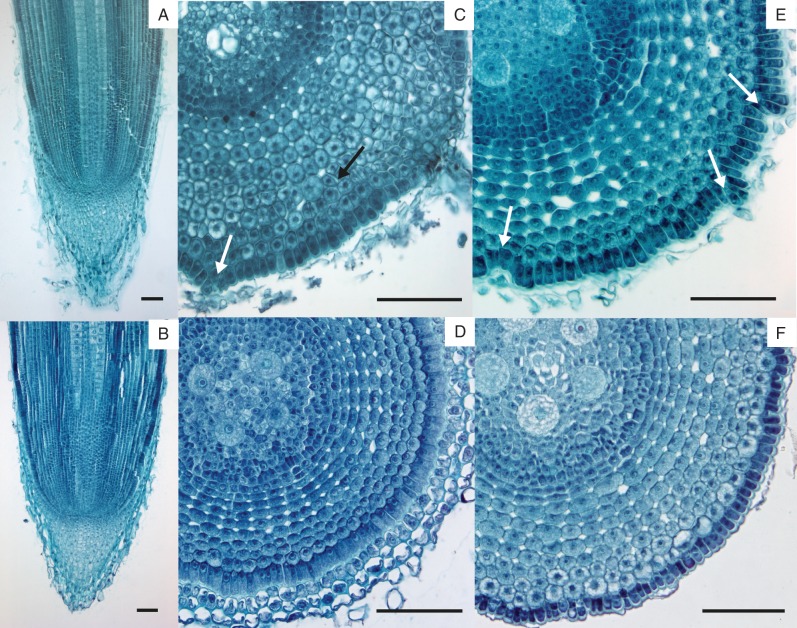
Unlike in LRs, the closed arrangement of lrt1 primary root apical meristems (Fig. 4A) apparently did not differ from the wild-type (Fig. 4B). However, the pattern of cell division seems to be affected up to some distance behind the promeristem, causing local irregularities in the radial and tangential arrangement of cortical cells (Fig. 4C). These are most evident in the outermost layers of the hypodermis and overlaying epidermis, especially in the subapical and older parts of the primary roots (Fig. 4E, compared with the wild-type in 4D, F).
Fig. 4.
Root apical meristem structure and irregularities in epidermal/cortical cell divisions behind the apex in primary roots of lrt1. The mutant shows normal arrangement of the closed primary root apical meristem (A) similar to the wild-type (B). In some places, the mutant displays disruption of the regular radial and tangential organization of the cortex (C, black arrow) and epidermis (C, white arrow). White arrows in C and E point to particular sites of disturbances at different distances from the meristem in lrt1 (C: approx. 300 µm, E: approx. 700 µm). None of these differences was found in the wild-type (D: approx. 300 µm, F: approx. 800 µm). Aerated hydroponics, permanent serial sections from four root tips 1 cm long were stained with Safranine and Fast Green. Scale bars = 100 µm, except (C) = 150 µm.
The lrt1 affects the function of surface layers of the primary root
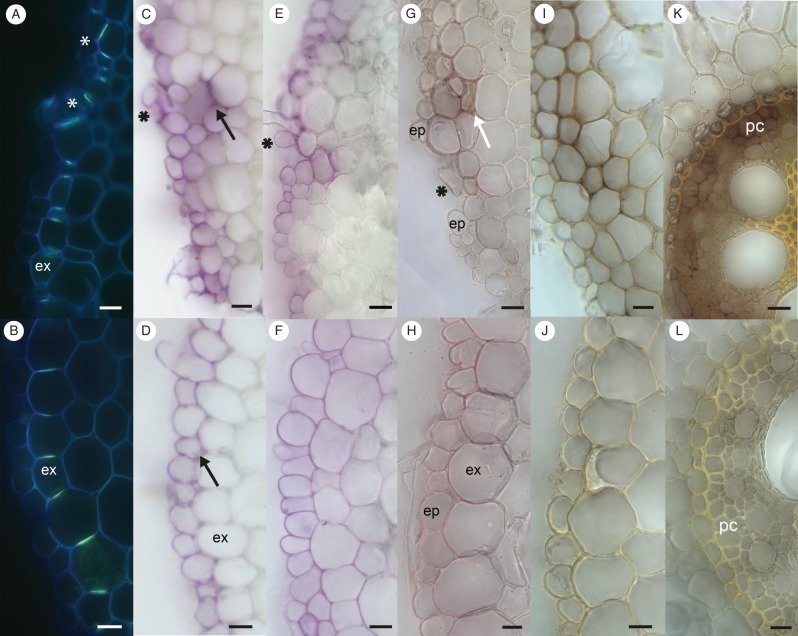
The exodermis, suberized hypodermal layer with Berberin stainable Casparian strips, was only detected in those roots of plants cultivated between filter paper (Fig. 5A, B). An incorrect pattern of cell division within the subepidermal layers of lrt1 mutants impaired the correct development of exodermal cell-wall modifications, and the proper positioning of the Casparian strips (Fig. 5A), which normally form a continuous cylinder (Fig. 5B). Frequently, cell-wall adhesion was reduced in the surface layers, causing partial separation of individual cells from layers predominantly observed in the older parts of mutant primary root (Fig. 5C, G). Irregularities in the arrangement of the exodermis modified the pattern of periodic acid penetration. This tracer penetrated sites of defective exodermis arrangement, and marked underlying cell layers of the cortex. The sites where the probe did not pass across the exodermis (Fig. 5C) were similar to the continuous exodermis of wild-type seedlings (Fig. 5D). Prolonged exposure to periodic acid did not have any significant effect on the depth of tracer penetration, which stopped in sites of irregularities approximately in the third layer below the lrt1 epidermis (data not shown). Ectopic lignification was detected in sites of exodermal irregularities in lrt1 roots (Fig. 5G, compared with the wild-type in Fig. 5H). The absence of a developed exodermis from roots cultivated in hydroponics (Fig. 5E, F) allowed deep penetration of the periodic acid into wild-type roots (Fig. 5F). By contrast, in mutant roots under the same cultivation conditions, the probe did not penetrate into the root, only reaching the epidermis, and to sites where the irregular cell arrangement passed two layers deeper (Fig. 5E). The outermost cortical cell layers of lrt1 were positive for lignin, but only in patches for suberin; and no Berberin staining of Casparian strips was detected. Therefore, their pattern did not correspond to the arrangement of a typical exodermis; however, the permeability test strongly resembled the distribution of the probe in lrt1 roots cultivated in moist paper (Fig. 5G).
Fig. 5.
Details of primary root surface layers of 16-d-old wild-type and lrt1 plants. (A, B) Exodermis (ex) only developed by cultivation in paper. Arrangement of Casparian strips is modified in lrt1 (A, asterisk) in comparison with the wild-type (B), where Casparian strips form a continuous cylinder. Berberine hemisulfate with Crystal Violet, UV. (C–F) The permeability test of the root surface with periodic acid detected changes in exodermis function in lrt1. Pink colour indicates tissues penetrated with the probe. The plants were cultivated in paper (C, D) and in non-aerated hydroponics (E, F), where the roots created no exodermis. The lrt1 mutant shows the same pattern of penetration under both cultivation conditions (C, E). In all cases, periodic acid did not penetrate beyond the epidermis, but in sites of irregularities moved approximately into the third layer from the surface (black arrow). In wild-type primary roots, the probe only penetrated into the epidermis in paper-cultivated roots (D, black arrow), while in non-aerated hydroponics it penetrated into the whole cortex (F). (G, H) Lignin detection with HCl-phloroglucinol in paper-cultivated roots. In lrt1 roots (G) lignin was deposited in the peripheral layers of the primary root, mainly in sites of irregular cell arrangement (white arrow; ep, epidermis), similar to the penetration of periodic acid. In wild-type primary roots, lignin was detected in the exodermis (ex) (H). Cell-wall adhesion is weakened in the surface layers of lrt1, causing a release of individual cells from the layers (C, E, G, asterisks). (I–L) Detection of peroxidase activity in paper-cultivated plants. Strong activity of peroxidase was detected in all tissues of lrt1 roots (I, K). The maximum activity was observed in surface layers of the cortex (I), and in the central cylinder, mainly in the pericycle (pc) (K). Very low activity was observed in wild-type primary roots (J, L). All sections were cut in the middle of the primary root. Scale bars = 20 µm.
Peroxidase activity and lignin content
Higher peroxidase activity was detected in the root tissues 1 cm from the tip (data not shown) and in the middle part of lrt1 roots grown under any of the cultivation conditions. The highest peroxidase activity was recorded with the paper cultivation (Fig. 5I–L). The strongest response was observed in the peripheral layers of the lrt1 cortex (Fig. 5I), and in the pericycle between protoxylem poles (Fig. 5K) as comparing with the wild-type (Fig. 5J and L, respectively).
While induced lignification was frequently recorded in roots of lrt1 plants, no significant quantitative changes in overall lignin content were detected (Supplementary Data Fig. S3). The cell walls of metaxylem vessels, pericycle and pith were significantly thicker in lrt1 mutants (Supplementary Data Table S2). The mutant roots had a higher mechanical resistance during sectioning, and displayed an increased resistance of the cortical tissues to pectinase and cellulase (data not shown).
DISCUSSION
The root system of maize (Zea mays) contains several types of morphologically distinct roots (Hochholdinger and Tuberosa, 2009): embryonic roots – the primary root, and several seminal roots; as well as postembryonic nodal roots – underground crown roots and brace roots from shoot nodes (Feldman, 1994). LRs further extend the absorptive surface of the lower orders of roots. The maize mutant lrt1 was described as not initiating LRs during early postembryonic development (6 d after germination), when grown in germination paper rolls soaked with distilled water (Hochholdinger and Feix, 1998). Partial recovery was reported with arbuscular mycorrhiza and/or high phosphate (Paszkowski and Boller, 2002). Previous proteomic studies had detected a set of proteins that were preferentially expressed in lrt1 roots; a subset of these proteins is related to lignin metabolism (Hochholdinger et al., 2004a). The current study was initiated to understand more about the role of lrt1 in root development and growth. Because the effects of environmental conditions had been recorded previously, different cultivation systems and long-term cultivations were involved to allow for better generalization of the results.
The frequency of initiation events was quantified in later plant development to further characterize the lrt1 effect. Bulges of LRP appeared on the surface of primary and seminal roots of 6-d-old lrt1 seedlings under hydroponics, appearing 2 days later in those grown on moist paper. However, bulges of LRP did not typically emerge out of the primary root, suggesting a substantial role of lrt1 in the later development of LRs. Quantitative analysis of initiation indicated that the primary target of lrt1 is not initiation per se, as originally suggested. The density of initiation was normalized to different cortex cell length in lrt1 mutants, and expressed as an LR initiation index (Dubrovsky et al., 2009). In fact, the total number of initiated LRP within the primary root was not significantly different from the wild-type in stagnant hydroponics, and was even slightly higher in aerated hydroponics. The difference between the current and original observations of the lack of LRP in early postembryonic development (Hochholdinger and Feix, 1998) should be related to the effects of cultivation conditions (e.g. different types of cultivation paper; different nutrient solution), as well as the ages of the sampled roots. Due to the augmented sensitivity of lrt1 plants to environmental conditions, initiation was delayed and sampling for anatomical sections at 4 d did not reveal the presence of LRP. Older plantlets from the original experiment (figure 1c, d in Hochholdinger and Feix, 1998) were grown for 2 weeks in paper rolls before being transferred to hydroponics. Anatomical analyses were not performed on older roots (Hochholdinger and Feix, 1998) and the data presented thus expand on previous findings.
Initiation of LRs in maize takes place in pericycle sectors, opposite to the phloem, at some distance from the apical meristem (Bell and McCully, 1970). The process, which is similar to that in other plants such as A. thaliana, contains the determination of founder cells (De Smet, 2012), subsequent divisions and emergence of LRs from the primary root (Smith and De Smet, 2012). Defects related to lrt1 in the early stages of LRP included the formation of groups of dividing cells in the pericycle. This might be related to incorrect spatial regulation of the initiation events (Laskowski et al., 1995; Sreevidya et al., 2010), and/or the spatial definition of groups of dividing cells of LRP within the pericycle (Shuai et al., 2002).
A highly regular pattern of cell division is a typical feature of LRP development in many model plants (De Smet et al., 2006) such as: A. thaliana (Malamy and Benfey, 1997), Raphanus sativus (Szymanowska-Puľka and Nakielski, 2010), Vicia faba, Pisum sativum, Phaseolus vulgaris and Z. mays (Bell and McCully, 1970; MacLeod and Thompson, 1979). In maize, a set of unequal and equal divisions creates distinct LR populations of precursors present for stele, cortex with root epidermis, and root cap. Such a pattern is disturbed in lrt1 mutants, appearing more obvious in the later developmental stages. The means of differentiation of the primary root epidermis should also affect emergence of LRs. Penetration of the LRs through the overlying tissues is facilitated by local auxin maxima inducing the expression of cell-wall-remodelling enzymes, leading to cell-wall adhesion in A. thaliana (Péret et al., 2009). The typical separation of cell walls in the vicinity of the emerging LRs, and facilitating their emergence, is observed in the wild-type but not in lrt1 roots. Apparently, LRPs of lrt1 come through 'unprepared' layers of parental root tissues that do not easily open, and are stretched during LRP outgrowth. Moreover, lignification detected in the surface layers of primary roots may play an important role, causing difficulties during penetration of LRP, as described in O. sativa (Justin and Armstrong, 1991). However, the biomechanical effect of modified epidermal and hypodermal development can hardly explain the changes of LRP structure before emergence, or of LR apical meristems after emergence (Potocka et al., 2011). The premature loss of apical meristem activity of LRs should therefore (at least partially) be related to failure mechanisms of apical meristem maintenance. The loss of LR apical meristems was described earlier in maize as a common developmental event of some LRs in older parts of the primary root (Varney and McCully, 1991). Additionally, under unfavourable conditions, the root apical meristem can be reorganized, and its activity modified through redox-mediated signalling (De Tullio et al., 2010). Such an association seems to be attractive for lrt1, which is more sensitive to the environmental conditions. We can speculate that higher peroxidase activity (or other reactive oxygen species-related metabolism) in the tissues could further accelerate the onset of meristem activity termination of LRs. Impaired specification of cell lineages and the coordination of tissue development is particularly obvious on precursors of the epidermis in LRP, with anomalous vacuolization and volume growth, which becomes far more distinct after emergence of the LRs (when epidermal cells with abnormal volume growth are particularly conspicuous).
Only a low percentage of LRs emerge in lrt1, and exceed a length of more than 0·5 mm. The emerged lrt1 LRs are shorter, curved and thicker, and the structure of their apex is intensely affected. Loss of the root apical meristem probably eliminates the inhibitory effect, and the formation of secondary LRs very close to the root tip is frequently observed.
The development of primary roots is also affected with mutation; noticeable changes in their length and radial growth were recorded. Contrary to LRs, where the apical meristem structure, as well as its function are severely affected; no significant defects in the longitudinal arrangement and maintenance of the apical meristem were observed in the primary roots, pointing to different regulatory mechanisms and/or responses. Such differences in regulatory mechanisms for different types of roots were reported earlier for other maize mutant lines (Hochholdinger et al., 2004b; Inukai et al., 2005). Irregularities connected to an altered manner of differentiation and/or aberrant cell divisions in the basal part (some distance from the promeristem) of ground meristem and protoderm were evident in differentiated parts of lrt1 primary roots. The higher variation of cell sizes indicates some impaired coordination of development, partial loss of cell adhesion and continuity of layers, all of which results in disruption of the root surface. These disturbances could be connected to ectopically induced lignification; however, it is difficult here to separate cause and consequence. Plant extracellular peroxidases play important roles during the plant's entire development (Hiraga et al., 2001); however, they are also induced during plant injury and defence responses, and they play a key role in several metabolic processes, for example auxin metabolism, lignin and suberin formation, cross-linking of cell-wall components, and the metabolism of reactive oxygen species (Almagro et al., 2009; Burr and Fry, 2009). Peroxidases use H2O2 for oxidation and connection of cell-wall compounds such as tyrosine residues, monolignols, suberin units and ferulic acids, resulting in the creation of a rigid network (Passardi et al., 2004). The higher activity of peroxidases in lrt1 might be connected to the higher (and possibly premature) rigidity of the cell walls of developing tissues. It might be related to the thicker cell walls of lrt1, together with the higher mechanical resistance of tissues, increased resistance of cortical tissues to pectinase and cellulase, and disrupted coordination of volume changes during growth of the outer cell layers. In this case, further work would also be necessary to distinguish whether the higher abundance of peroxidase and the mentioned cell-wall modifications are the mechanisms creating the damage, or whether it is a consequence/response to higher stress (suboptimal conditions) in the tissue.
In maize, under certain environmental conditions, the hypodermal layers differentiate (in our study under paper cultivation) into exodermis – morphologically distinct cell layers with Casparian strips and suberin lamellae (Enstone and Peterson, 2005; Zimmermann et al., 2000), which modulates the permeability of the root surface, and accordingly also in their interactions with the surrounding rhizosphere (Hose et al., 2001). Both the proper spatial arrangement and coordination of exodermal cell development are required for the correct set-up of a continual cylinder of Casparian strips, as well as its later functioning. A similar phenomenon was described in the endodermis of A. thaliana (Martinka et al., 2012). In lrt1 roots, the improperly coordinated divisions lead to the loss of continuity of the exodermal cell layer, and a disturbance in the positioning of Casparian strips. The effect of lost barrier continuity was documented by penetration of an apoplastic tracer (periodic acid), which locally passed across the exodermis of lrt1 but not the wild-type. The penetration depth of the apoplastic tracer in lrt1 was restricted to layers beneath due to ectopic lignification, suberization and other cell-wall modifications. The presence of an apoplastic barrier was also detected in lrt1 roots under conditions non-inducible for the exodermis in the wild-type. Penetration, in this case, was limited to the outermost cortical layers; in the wild-type, without exodermis grown under the same cultivation conditions, the apoplastic tracer passed up to the endodermis. The character of this barrier in lrt1 does not fit the standard exodermis. Cell-wall modifications resesmble those induced by injury, which include inter alia ectopic deposition of lignin and patchy suberization reducing apoplastic permeability of non-exodermal layers.
Higher levels of the enzymes of lignin biosynthesis and the metabolism of phenolics detected in proteomic studies of lrt1 roots (Hochholdinger et al., 2004a) might be related to such ‘injury-related' responses, as well as to the abnormal lignification in the pericycle or outer cortex. Such protection/defence reactions are in accordance with the higher sensitivity of lrt1 to environmental conditions, and possibly its lower ability to control the internal environment. To confirm whether lignin biosynthesis is related to local stress or injury responses, or if it represents a global metabolic change in lrt1 plants, we have quantified the lignin content in different plant organs. No significant increase was detected in lrt1, indicating that a global increase of lignin metabolism is not an effect of lrt1. Most probably, it is a correction mechanisms responding to the internal tissue imbalance in the coordination of both development and differentiation.
Based on the results of this study, the lrt1 gene takes part in the control of LRP development and emergence, and also disturbs the ability of plants to maintain the activity of LR apical meristems. Furthermore, the mutant indicates a difference of root apical meristem development and maintenance mechanisms within the primary roots and LRs. Hence, lrt1 provides a unique link between LR formation, root apical meristem maintenance and their environment. Identification of the lrt1 gene sequence will provide further insights into LR initiation and regulation of development in monocot plants.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We wish to acknowledge the help of Dr A. Černíková (Inst. Applied Mathematics and IT, Faculty of Science, Charles University in Prague) with the statistical evaluation of our data. Financial support was provided by projects MŠMT LC06034, COST 49-208081 and GAUK 43-251346.
LITERATURE CITED
- Almagro L, Ros LVG, Belchi-Navarro S, Bru R, Barceló AR, Pedreńo MA. Class III peroxidases in plant defence reactions. Journal of Experimental Botany. 2009;60:377–390. doi: 10.1093/jxb/ern277. [DOI] [PubMed] [Google Scholar]
- Arnon DI. Microelements in culture-solution experiments with higher plants. American Journal of Botany. 1938;25:322–325. [Google Scholar]
- Bell D, McCully ME. A histological study of lateral root initiation in Zea mays. Protoplasma. 1970;70:179–205. [Google Scholar]
- Bruce RJ, West CA. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiology. 1989;91:889–897. doi: 10.1104/pp.91.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundrett MC, Enstone DE, Peterson CA. A berberine-aniline blue fluorescent staining procedure for suberin, lignin and callose in plant tissue. Protoplasma. 1988;146:133–142. [Google Scholar]
- Brundrett MC, Kendrick B, Peterson CA. Efficient lipid staining in plant material with Sudan red 7B or Fluorol yellow 088 in polyethylene glycol-clycerol. Biotechnic and Histochemistry. 1991;66:111–116. doi: 10.3109/10520299109110562. [DOI] [PubMed] [Google Scholar]
- Burr SJ, Fry SC. Feruloylated arabinoxylans are oxidatively cross-linked by extracellular maize peroxidase but not by horseradish peroxidase. Molecular Plant. 2009;2:883–892. doi: 10.1093/mp/ssp044. [DOI] [PubMed] [Google Scholar]
- Chhun T, Taketa S, Tsurumi S, Ichii M. Interaction between two auxin-resistant mutants and their effects on lateral root formation in rice (Oryza sativa L.) Journal of Experimental Botany. 2003a;54:2701–2708. doi: 10.1093/jxb/erg306. [DOI] [PubMed] [Google Scholar]
- Chhun T, Taketa S, Tsurumi S, Ichii M. The effects of auxin on lateral root initiation and root gravitropism in a lateral rootless mutant Lrt1 of rice (Oryza sativa L.) Plant Growth Regulation. 2003b;39:161–170. [Google Scholar]
- Clowes FAL. Chimeras and the origin of lateral root primordia in Zea mays. Annals of Botany. 1978;42:801–807. [Google Scholar]
- De Smet I. Lateral root initiation: one step at a time. New Phytologist. 2012;193:867–873. doi: 10.1111/j.1469-8137.2011.03996.x. [DOI] [PubMed] [Google Scholar]
- De Smet I, Vanneste S, Inze D, Beeckman T. Lateral root initiation or the birth of a new meristem. Plant Molecular Biology. 2006;60:871–887. doi: 10.1007/s11103-005-4547-2. [DOI] [PubMed] [Google Scholar]
- De Tullio MC, Jiang K, Feldman LJ. Redox regulation of root apical meristem organization: connecting root development to its environment. Plant Physiology and Biochemistry. 2010;48:328–336. doi: 10.1016/j.plaphy.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Soukup A, Napsucialy-Mendivil S, Jeknić Z, Ivanchenko MG. The lateral root initiation index: an integrative measure of primordium formation. Annals of Botany. 2009;103:807–817. doi: 10.1093/aob/mcn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstone DE, Peterson CA. Suberin lamella development in maize seedling roots grown in aerated and stagnant conditions. Plant, Cell and Environment. 2005;28:444–455. [Google Scholar]
- Esau K. Anatomy of seed plants. New York: Wiley; 1965. [Google Scholar]
- Feldman L. The maize root. In: Freeling M, Walbot V, editors. The maize handbook. New York: Springer; 1994. pp. 29–33. [Google Scholar]
- Hao Z, Masahiko I. A mutant RM109 of rice (Oryza sativa L.) exhibiting altered lateral root initiation and gravitropism. Japanese Journal of Crop Science. 1999;68:245–252. [Google Scholar]
- Hiraga S, Sasaki K, Hiroyuki I, Ohashi Y, Matsui H. A large family of class III plant peroxidases. Plant Cell Physiology. 2001;42:462–468. doi: 10.1093/pcp/pce061. [DOI] [PubMed] [Google Scholar]
- Hoagland DR, Amon DI. The water-culture method for growing plants without soil. Circular 347 of University of California, Agricultural Experimental Station Berkeley. 1950 [Google Scholar]
- Hochholdinger F, Feix G. Early post-embryonic root formation is specifically affected in the maize mutant lrt1. The Plant Journal. 1998;16:247–255. doi: 10.1046/j.1365-313x.1998.00280.x. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Tuberosa R. Genetic and genomic dissection of maize root development and architecture. Current Opinion in Plant Biology. 2009;12:172–177. doi: 10.1016/j.pbi.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Park WJ, Feix GH. Cooperative action of SLR1 and SLR2 is required for lateral root-specific cell elongation in maize. Plant Physiology. 2001;125:1529–1539. doi: 10.1104/pp.125.3.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochholdinger F, Guo L, Schnable PS. Lateral roots affect the proteome of the primary root of maize (Zea mays L.) Plant Molecular Biology. 2004a;56:397–412. doi: 10.1007/s11103-004-3476-9. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Woll K, Sauer M, Dembinsky D. Genetic dissection of root formation in maize (Zea mays) reveals root-type specific developmental programmes. Annals of Botany. 2004b;93:359–368. doi: 10.1093/aob/mch056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Hose E, Clarkson DT, Steudle E, Schreiber L, Hartung W. The exodermis: a variable apoplastic barrier. Journal of Experimental Botany. 2001;52:2245–2264. doi: 10.1093/jexbot/52.365.2245. [DOI] [PubMed] [Google Scholar]
- Hotelling H. The generalization of Student's ratio. Annals of Mathematical Statistics. 1931;2:360–378. [Google Scholar]
- Inukai Y, Sakamoto T, Ueguchi-Tanaka M, et al. Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signalling. The Plant Cell. 2005;17:1387–1396. doi: 10.1105/tpc.105.030981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen L, Roberts I, De Rycke R, Beeckman T. Phloem-associated auxin response maxima determine radial positioning of lateral roots in maize. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367:1525–1533. doi: 10.1098/rstb.2011.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen WA. Botanical histochemistry – principles and practice. San Francisco: Freeman; 1962. [Google Scholar]
- Johansen DA. Plant microtechnique. New York: McGraw-Hill; 1940. [Google Scholar]
- Justin SHFW, Armstrong W. Evidence for the involvement of ethene in aerenchyma formation in adventitious roots of rice (Oryza sativa L.) New Phytologist. 1991;118:49–62. [Google Scholar]
- Kapulnik Y, Delaus PM, Resnick N, et al. Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta. 2011;233:209–216. doi: 10.1007/s00425-010-1310-y. [DOI] [PubMed] [Google Scholar]
- Lange BM, Lapierre C, Sandermann H. Elicitor-induced spruce stress lignin. Structural similarity to early developmental lignings. Plant Physiology. 1995;108:1277–1287. doi: 10.1104/pp.108.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski MJ, Williams ME, Nusbaum H, Sussex IM. Formation of lateral root meristems is a two-stage process. Development. 1995;121:3303–3310. doi: 10.1242/dev.121.10.3303. [DOI] [PubMed] [Google Scholar]
- Lavy M, Prigge MJ, Tigyi K, Estelle M. The cyclophilin DIAGEOTROPICA has a conserved role in auxin signaling. Development. 2012;139:1115–1124. doi: 10.1242/dev.074831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenochová Z, Soukup A, Votrubová O. Aerenchyma formation in maize roots. Biologia Plantarum. 2009;53:263–270. [Google Scholar]
- MacLeod RD, Thompson A. Development of lateral root primordia in Vicia faba, Pisum sativum, Zea mays and Phaseolus vulgaris: rates of primordium formation and cell doubling times. Annals of Botany. 1979;44:435–449. [Google Scholar]
- Majer Ch, Hochholdinger F. Defining the boundaries: structure and function of LOB domain proteins. Trends in Plant Science. 2011;16:47–52. doi: 10.1016/j.tplants.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Malamy JE. Intrinsic and environmental response pathways that regulate root system architecture. Plant, Cell and Environment. 2005;28:67–77. doi: 10.1111/j.1365-3040.2005.01306.x. [DOI] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
- Martinka M, Dolan L, Pernas M, Abe J, Lux A. Endodermal cell–cell contact is required for the spatial control of Casparian band development in Arabidopsis thaliana. Annals of Botany. 2012;110:361–371. doi: 10.1093/aob/mcs110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday GK, Lomax TL, Rayle DL. Characterization of the growth and auxin physiology of roots of the tomato mutant, diageotropica. Planta. 1995;195:548–553. doi: 10.1007/BF00195714. [DOI] [PubMed] [Google Scholar]
- Park WJ, Hochholdinger F, Gierl A. Release of the benzoxazinoids defense molecules during lateral- and crown root emergence in Zea mays. Journal of Plant Physiology. 2004;161:981–985. doi: 10.1016/j.jplph.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Passardi F, Penel C, Dunand Ch. Performing the paradoxical: how plant peroxidases modify the cell wall. Trends in Plant Science. 2004;9:534–540. doi: 10.1016/j.tplants.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Paszkowski U, Boller T. The growth defect of lrt1, a maize mutant lacking lateral roots, can be complemented by symbiotic fungi or high phosphate nutrition. Planta. 2002;214:584–590. doi: 10.1007/s004250100642. [DOI] [PubMed] [Google Scholar]
- Pearse AG. London: J. and A. Churchill; 1968. Histochemistry (theoretical and applied) [Google Scholar]
- Péret B, Larrieu A, Bennett MJ. Lateral root emergence: a difficult birth. Journal of Experimental Botany. 2009;60:3637–3643. doi: 10.1093/jxb/erp232. [DOI] [PubMed] [Google Scholar]
- Potocka I, Szymanowska-Puľka J, Karczewski J, Nakielski J. Effect of mechanical stress on Zea root apex. I. Mechanical stress leads to the switch from closed to open meristem organization. Journal of Experimental Botany. 2011;62:4583–4593. doi: 10.1093/jxb/err169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht M, Strnad M, Scanlon MJ, et al. Auxin immunolocalization implicates vesicular neurotransmitter-like mode of polar auxin transport in root apices. Plant Signaling and Behavior. 2006;1:122–133. doi: 10.4161/psb.1.3.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvendran RR. Analysis of cell wall material from plant tissues: extraction and purification. Phytochemistry. 1975;14:1011–1017. [Google Scholar]
- Shuai B, Reynaga-Peňa CG, Springer PS. The LATERAL ORGAN BOUNDARIES gene defines a novel, plant-specific gene family. Plant Physiology. 2002;129:747–761. doi: 10.1104/pp.010926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, De Smet I. Root system architecture: insights from Arabidopsis and cereal crops. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367:1441–1452. doi: 10.1098/rstb.2011.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukup A, Armstrong W, Schreiber L, Franke R, Votrubová O. Apoplastic barriers to radial oxygen loss and solute penetration: a chemical and functional comparison of the exodermis of two wetland species, Phragmites australis and Glyceria maxima. New Phytologist. 2007;173:264–278. doi: 10.1111/j.1469-8137.2006.01907.x. [DOI] [PubMed] [Google Scholar]
- Sreevidya VS, Hernandez-Oane RJ, Gyaneshwar P, Lara-Flores M. Changes in auxin distribution patterns during lateral root development in rice. Plant Science. 2010;178:531–538. [Google Scholar]
- Szymanowska-Puľka J, Nakielski J. The tensor-based model for growth and cell divisions of the root apex. II. Lateral root formation. Planta. 2010;232:1207–1218. doi: 10.1007/s00425-010-1239-1. [DOI] [PubMed] [Google Scholar]
- Tung P, Hooker TS, Tampe PA, Reid DM, Thorpe TA. Jasmonic acid: effects on growth and development of isolated tomato roots cultured in vitro. International Journal of Plant Sciences. 1996;157:713–721. [Google Scholar]
- Varney GT, McCully ME. The branch roots of Zea. II. Developmental loss of the apical meristem in field-grown roots. New Phytologist. 1991;118:535–546. [Google Scholar]
- Wang H, Taketa S, Miyao A, Hirochika H, Ichii M. Isolation of a novel lateral-rootless mutant in rice (Oryza sativa L.) with reduced sensitivity to auxin. Plant Science. 2006;170:70–77. [Google Scholar]
- Woll K, Borsuk LA, Stransky H, Nettleton D, Schnable PS, Hochholdinger F. Isolation, characterization, and pericycle-specific transcriptome analyses of the novel maize lateral and seminal root initiation mutant rum1. Plant Physiology. 2005;139:1255–1267. doi: 10.1104/pp.105.067330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann HM, Hartmann K, Schreiber L, Steudle E. Chemical composition of apoplastic transport barriers in relation to radial hydraulic conductivity of corn roots (Zea mays L.) Planta. 2000;210:302–311. doi: 10.1007/PL00008138. [DOI] [PubMed] [Google Scholar]
- Zimmermann R, Sakai H, Hochholdinger F. The gibberelic acid stimulated-like gene family in maize and its role in lateral root development. Plant Physiology. 2010;152:356–365. doi: 10.1104/pp.109.149054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.