Abstract
Background
Electrical capacitance, measured between an electrode inserted at the base of a plant and an electrode in the rooting substrate, is often linearly correlated with root mass. Electrical capacitance has often been used as an assay for root mass, and is conventionally interpreted using an electrical model in which roots behave as cylindrical capacitors wired in parallel. Recent experiments in hydroponics show that this interpretation is incorrect and a new model has been proposed. Here, the new model is tested in solid substrates.
Methods
The capacitances of compost and soil were determined as a function of water content, and the capacitances of cereal plants growing in sand or potting compost in the glasshouse, or in the field, were measured under contrasting irrigation regimes.
Key Results
Capacitances of compost and soil increased with increasing water content. At water contents approaching field capacity, compost and soil had capacitances at least an order of magnitude greater than those of plant tissues. For plants growing in solid substrates, wetting the substrate locally around the stem base was both necessary and sufficient to record maximum capacitance, which was correlated with stem cross-sectional area: capacitance of excised stem tissue equalled that of the plant in wet soil. Capacitance measured between two electrodes could be modelled as an electrical circuit in which component capacitors (plant tissue or rooting substrate) are wired in series.
Conclusions
The results were consistent with the new physical interpretation of plant capacitance. Substrate capacitance and plant capacitance combine according to standard physical laws. For plants growing in wet substrate, the capacitance measured is largely determined by the tissue between the surface of the substrate and the electrode attached to the plant. Whilst the measured capacitance can, in some circumstances, be correlated with root mass, it is not a direct assay of root mass.
Keywords: Barley, Hordeum vulgare, soil capacitance, root mass, electrical circuit, root phenomics, wheat, Triticum aestivum
INTRODUCTION
There has been recent interest in the use of electrical techniques for quantifying root systems (Cao et al., 2010, 2011; Urban et al., 2011; Dietrich et al., 2012; Ellis et al., 2012). Many studies have reported good correlations between root mass and electrical capacitance, measured between an electrode inserted at the base of the stem and an electrode in the rooting substrate (e.g. Chloupek, 1977; Dalton, 1995; van Beem et al., 1998; Preston et al., 2004; Ozier-Lafontaine and Bajazet, 2005; McBride et al., 2008; Tsukahara et al., 2009). Linear relationships between root mass and electrical capacitance have been interpreted using an electrical model proposing that roots behave as cylindrical capacitors and their capacitances can be added together as though wired in parallel (Dalton, 1995). This model was tested in hydroponics by Dietrich et al. (2012), who found that the capacitance of barley (Hordeum vulgare) appeared to be determined, not by the mass of their root system, but by the cross-sectional area of roots at the solution surface. These authors also observed (i) that capacitance was not linearly related to the mass of roots in solution when root systems were partly submerged and (ii) that excising the root below the solution surface had a negligible effect on the capacitance measured. These observations are inconsistent with the model of Dalton (1995). A new model for plant capacitance was proposed by Dietrich et al. (2012), suggesting that plant tissue behaves as a continuous dielectric and, provided the capacitance of the tissue is much smaller than that of the rooting substrate, the capacitance measured in hydroponics is dominated by tissue between the solution surface and the electrode attached to the plant. The new model suggests that the measured capacitance will be inversely proportional to the distance between the plant electrode and the solution surface. This model remains to be tested in solid rooting substrates, where both the capacitance of the substrate and the contact between roots and solution are likely to be smaller than in hydroponics and will vary with the water content of the rooting substrate. In this study, the ability of the new model to explain capacitance measurements made on cereals growing in sand or in compost in the glasshouse, or in a sandy-loam soil in the field, was tested under various water regimes.
MATERIALS AND METHODS
Experiment 1: capacitances of compost and soil
The capacitances of compost and soil were measured using a two-terminal LCR Meter (Extech 380193; Extech Instruments, Waltham, MA, USA) in the laboratory at a range of water contents, using 16·5 cm long stainless steel rods (diameter 3 mm) as parallel electrodes separated by up to 40 cm. Compost (approx. 0·85 v/v peat, 0·1 v/v sand, 0·05 v/v vermiculite) contained 1 kg m−3 of cellulose-based water management additive (Celcote, Certis, Wiltshire, UK), 2·5 kg m−3 of a 1:1 calcium–magnesium limed mix, and 4·25 kg m−3 of NPK-fertilizer (Osmocote ‘Exact Hi Start, 5-6M’, Scotts, Baulkham Hills, Australia). Soil was collected from East Loan Field (latitude 56·4560 °N, longitude 3·0800 °W), The James Hutton Institute (JHI), Dundee, UK. Compost or field soil was placed in a plastic container (60 cm long × 40 cm wide × 11 cm deep) with drainage holes and irrigated to a water content approaching field capacity. Nine rod electrodes were inserted into the compost or soil in a line. The compost or field soil was then allowed to dry for 65 d and capacitance and water content were measured periodically at five locations in the substrate. The volumetric water content of the compost or soil was measured using a theta probe (ML2x, Delta-T Devices, Cambridge, UK). Soil capacitance was also measured in the field as a function of electrode separation using steel rod electrodes, at a soil water content of 0·223 cm3 cm−3, 30 min after rain.
Experiment 2: wheat (Triticum aestivum) grown in sand in the glasshouse
Seeds of 40 cultivars of winter wheat were imbibed for 3–5 h in water and then sterilized in a solution of 2 % calcium hypochlorite for 15 min. Sterilized seeds were rinsed in distilled water and placed between sheets of moist filter paper in Petri dishes. The Petri dishes were covered with aluminium foil and incubated at a temperature of 4 °C for 7 d. Seedlings with similar leaf development were selected and transferred on 24 October 2008 to vertically aligned plastic tubes (1 m length, 5 cm diameter) lined with heavy-duty black plastic sheeting and filled with a gravel–grit–sand mixture (40:40:20 v/v/v, 6:7:4 w/w/w) over 0·1 m3 gravel. The bottom of each tube was covered with 0·5 mm pore size nylon mesh.
The tubes were arranged in 42 rows and 12 columns in a compartment of a Cambridge-type glasshouse at JHI, Dundee, UK (latitude 56·4566 °N, longitude 3·0708 °W). Four rows constituted a block. The experimental plants were completely surrounded by guard plants (‘Hereward’) occupying all tubes in rows 1 and 42 and columns 1 and 12. Individuals of each of 40 cultivars were randomly assigned to one of 40 tubes in each block. The compartment was set to maintain temperatures of 20 °C by day and 15 °C at night using automatic vents and supplementary heating. Daylight was supplemented by artificial lighting (MASTER SON-T PIA Green Power; Philips, Guildford, UK) to maintain an irradiance >200 W m−2 for 16 h each day.
Prior to the transfer of seedlings, all tubes were flushed with water delivered at 9 cm3 min−1 through drip feeders using a HortiMaX Irrigation Computer (Aqua 500; HortiMaX, Pijnacker, The Netherlands). Following the transfer of plants to tubes, each tube was fertigated daily at 0300 h for 3–6 min with a mineral solution containing 4·359 mm K+, 2·1 mm Ca2+, 2·0 mm NH4+, 0·75 mmM Mg2+, 10·0 µm FeNaEDTA, 1·0 µm Mn2+, 1·0 µm Zn2+, 0·25 µm Cu2+, 4·2 mm Cl−, 4·0 mm NO3−, 1·75 mm SO42–, 0·307 mm H2PO4−, 12·5 µm H2BO3 and 0·25 µm MoO42–, and weekly for 1–2 min with a solution of 2·1 mm CaCl2, both delivered at 9 cm3 min−1 using a HortiMax GPS Irrigation Computer. Solutions were supplied to the fertigation system through a Dosatron (DI 16; Dosatron International, Bordeaux, France).
Vernalization was achieved by moving tubes containing plants on 19 November 2008 to a growth chamber supplying 12 h light daily, running at 4 °C. Whilst plants were in the growth chamber, all tubes were placed in containers containing a pool of water 1 cm deep. Plants were removed from the growth chamber and returned to the glasshouse on 7 January 2009 and fertigation was resumed. Plants were harvested at commercial maturity, between 18 and 27 May 2009, when the grain moisture content approximated 8–10 % fresh mass.
At harvest, shoots were cut at the surface of the sand, and the base of the shoot plus roots remained in the sand. Selected sand columns were then irrigated with tap water until it flowed from the bottom of the tubes. Approximately 30 min after irrigation, when no water was pooled on the surface of the sand, a 16·5 cm long stainless steel rod electrode (diameter 3·2 mm) was inserted approx. 10 cm into the sand about 2·5 cm away from the base of a shoot. A second electrode, made from a stainless steel needle (NN-2325R, 0·6 × 25 mm Terumo, Leuven, Belgium), was inserted through the bases of the main stem and tillers. Electrodes were then connected to an Extech LCR Meter using the test leads supplied by the manufacturer. Capacitance was measured by applying 1 V at a frequency of 1 kHz. No difference was found in the relationships between root mass and capacitance measurements between LCR meters. Capacitance measurements were made on 1–5 replicate plants of 35 cultivars of winter wheat (A50-03, Alchemy, Avalon, Batis, Brompton, Caphorn, Claire, Cordiale, Deben, Dover, Einstein, Enorm, Flanders, Gatsby, Gladiator, Gulliver, Hereward, Isengrain, Lynx, Malacca, Maris Widgeon, Mascot, Monopol, Ochre, Opus, PBIS, Petrus, Rialto, Riband, Robigus, Scorpion 25, Soissons, Sokrates, Solstice and Zebedee). Roots were washed free of sand and their fresh mass was determined. Root material was dried at 70 °C in an oven for 3 d before their dry mass was determined.
Experiment 3: barley (Hordeum vulgare) grown in compost in the glasshouse
Seeds of barley (‘Optic’) were surface-sterilized using a solution of 2 % calcium hypochlorite for 15 min. Sterilized seeds were sown into plastic pots (height 20·5 cm, volume 3 L) each filled with 1·9 kg of the compost mixture described in Experiment 1 at a depth of 3 cm. Pots were placed in a glasshouse compartment at JHI on 30 September 2010 and watered daily. A polyethene mesh (9·5 threads mm−1, Tildenet, Bristol, UK) was used to retain the compost in the pots during inversion.
Capacitances of 43 plants were determined between 43 and 45 days after sowing (DAS). Capacitances were first measured using an Extech LCR Meter between a needle electrode inserted into the stem about 5 mm above the surface of the compost and a steel rod electrode in the compost. The capacitance of the stem tissue was then determined. To achieve this, the compost surface position was first marked on the stem of each plant with a waterproof pen. Then, stems were cut about 2 cm below the compost surface and removed from the compost. The capacitance was measured between an electrode contacting the stem at the compost surface mark and the original needle electrode site. The diameter of all tillers was determined. Shoot circumference and cross-sectional area of the hollow stems were calculated from perpendicular diameters of inner and outer surfaces at the position of the plant electrode and the soil surface. Calculations took into account that mature shoot pieces were elliptic and hollow. Thus the cross-sectional area was calculated by A = π(aobo – aibi) with a as the semi-major and b as the semi-minor axis, and the subscript indicates the inner or outer dimension of the hollow stem. Compost was washed off the root material, which was dried with a paper towel and weighed (EP214, Ohaus, Pine Brook, NJ, USA).
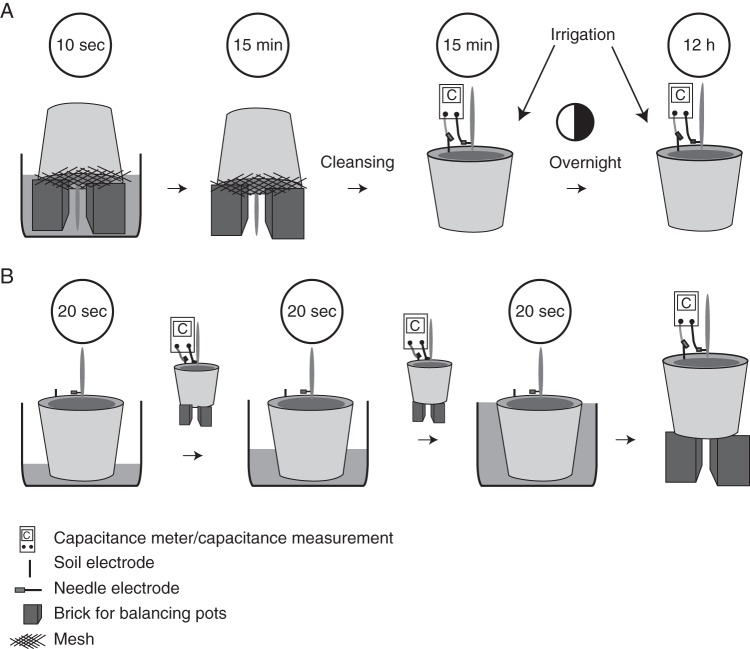
Watering was suspended for a further 70 plants from 45 DAS. Capacitance measurements were made on these plants between 65 and 75 DAS before, during and after the various controlled water treatments described in Fig. 1. The average height of the plant electrode above the compost was 5 mm. The position of the transition between wet and dry compost was observed using a snake camera (Model no. 8803AL, Goscam, Shezhen, China) in 14 pots that were furnished with a clear plastic tube marked with a scale (20 cm length, 5 cm diameter) for this purpose.
Fig. 1.
Controlled irrigation treatments performed in expt 3. Watering of compost-filled pots containing barley plants was stopped from 45 DAS and, at 65–75 DAS, the pots were randomly split into two groups. (A) One group of 35 pots were turned upside down and placed in a water-filled basin for 10 s. Bricks in the basin served as supports and ensured that only the first centimetre of the compost was wetted. Pots were then removed from the basin and placed upside down on bricks to drain for 15 min. Plants were then turned upright again and any compost adhering to the shoot was removed. Capacitance was then measured using an Extech LCR Meter between a needle electrode inserted into the stem of plants about 5 mm above the surface of the compost and a steel rod electrode in the compost. The pots were irrigated from above twice in the evening and once in the morning to a water content approaching field capacity. Capacitance was then remeasured. (B) The second group of 35 pots were placed in a basin which was then filled with water to a depth of 4 cm. After 20 s the pots were taken out and capacitance was measured using an Extech LCR Meter between a needle electrode inserted into the stem of plants about 5 mm above the surface of the compost and a steel rod electrode in the compost. Pots were returned to the basin, which was filled with water to a greater depth. After 20 s, pots were removed from the basin and capacitance was remeasured. This procedure was repeated until the water in the basin was level with the surface of the compost.
Experiment 4: barley grown in soil in the field
Winter barley (‘Siberia’) was grown in the field at JHI in 2010 and 2011. Seed was sown in East Loan Field (latitude 56·4560 °N, longitude 3·0800 °W) on 6 October 2009 and in East Pilmore Field (latitude 56·4577 °N, longitude 3·0718 °W) on 25 September 2010. Capacitance measurements were performed between 26 July and 14 August 2010, and on 20 August 2011. In 2010, capacitance was measured using an Extech LCR Meter between a needle electrode inserted into one or more tillers of a barley plant 1·5 cm above the ground and a steel rod electrode in the soil. The soil around the shoot was then irrigated with 10 cm3 of water and capacitance was measured 20 s later. This procedure was repeated until 100–200 cm3 of water had been added to the soil. In 2011, two steel rod electrodes were inserted in the soil close to pairs of neighbouring plants after irrigating the soil around each of the two plants with 100 cm3 of water and the soil between the two plants with 100 cm3 of water. The capacitance was then measured with the Extech LCR meter between different combinations of needle electrodes inserted in the tillers (either 3 mm or 1·5 cm above the soil surface) or the steel rod electrodes in the soil, as will be described in the Results section. This experiment was conducted on five plant pairs.
Statistics
Regressions were performed using Sigmaplot 12 software (Systat Software, Chicago, IL, USA). Data and regression coefficients are expressed as mean ± standard error (s.e.) from n determinations. In linear regressions, when the intercept did not differ significantly from zero, the regression was forced through the origin.
RESULTS AND DISCUSSION
Capacitances of compost and soil increase with water content
When measured with an electrode separation of 10 cm, the capacitances of both compost and field soil increased with increasing water content (Fig. 2A, B). This is consistent with previous studies (e.g. Robinson et al., 2005; Kizito et al., 2008; Wu et al., 2011). When capacitance was measured in compost at a water content of 0·447 cm3 cm−3, it decreased with increasing electrode separation (Fig. 2C). When capacitance was measured in soil in the laboratory at a water content of 0·263 cm3 cm−3 or in the field at a water content of 0·223 cm3 cm−3, it also decreased with increasing electrode separation (Fig. 2C). Soil capacitance measured in the field appeared to be inversely proportional to electrode separation (Fig. 2D).
Fig. 2.
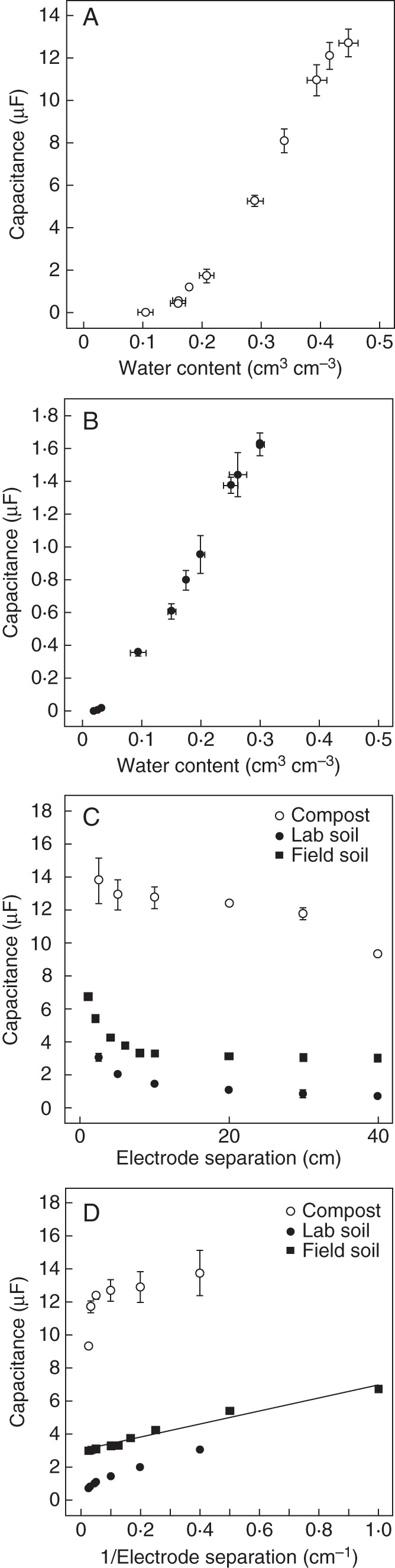
(A) Relationship between the capacitance of compost and its water content measured with an electrode separation of 10 cm. (B) Relationship between the capacitance of soil and its water content measured in the laboratory with an electrode separation of 10 cm. (C) Relationships between the capacitance of compost, of soil measured in the laboratory and of soil measured in the field (as indicated in the key) and electrode separation. (D) Relationships between the capacitance of compost, of soil measured in the laboratory and of soil measured in the field (as indicated in the key) and the reciprocal of electrode separation. In (C) and (D) capacitances were measured at water contents of 0·447 cm3 cm−3 for compost, 0·263 cm3 cm−3 for soil measured in the laboratory, and 0·223 cm3 cm−3 for soil measured in the field. Data for (A) and (B) represent means ± s.e. of five independent measurements of capacitance and water content. Data for (C) and (D) represent means ± s.e. of up to five independent measurements of capacitance. The linear regression in (D) is y = 4·00x + 3·00 (R2 = 0·967, n = 8 electrode separations).
Capacitance is correlated with root system mass in wheat grown in sand columns
In the sand column study (expt 2), a close linear relationship was found between the capacitance of wheat plants of different cultivars and root dry mass (Fig. 3). These data show that a linear relationship between capacitance and root mass can be obtained across many cereal genotypes in a sand culture system. However, it is possible that this relationship arises because of allometric relationships between root mass and other plant properties (cross-sectional area of roots near the surface of the sand, and dimensions of plant tissue between the surface of the sand and the electrode attached to the plant).
Fig. 3.
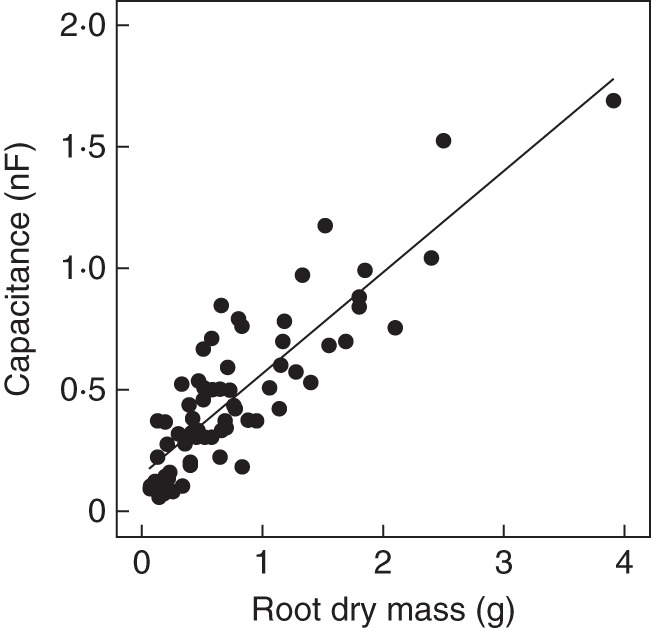
Relationship between the capacitance of wheat plants of different cultivars grown in sand columns and their root dry mass. The linear regression is y = 0·416x + 0·149 (n = 67, R2 = 0·753).
Maximal capacitance of barley plants requires local wetting of substrate around the base of the stem
The capacitance of barley plants growing in dry substrate was much smaller than in wet substrate (Fig. 4). Wetting either the top centimetre of the compost (expt 3; Fig. 1A) or the soil immediately around the shoot base with 1·0–2·5 cm3 of water (expt 4) sufficed to increase the capacitance to the values recorded in fully wetted soil (Fig. 4). Further wetting had only marginal effects on the capacitance. This is consistent with recommendations in the literature to perform capacitance measurements on plants in wet substrate (Chloupek, 1977; Kendall et al., 1982; Dalton, 1995; van Beem et al., 1998; McBride et al., 2008). Raising the water table in compost (Fig. 1B) had little effect on the capacitance of barley plants until the water table reached within 1–2 cm of the compost surface (Fig. 5A). Thus, wetting the substrate locally around the stem base is both necessary and sufficient to record the value of plant capacitance for a plant in thoroughly wetted soil (the usual measurement condition in the literature, e.g. Dalton et al., 1997).
Fig. 4.
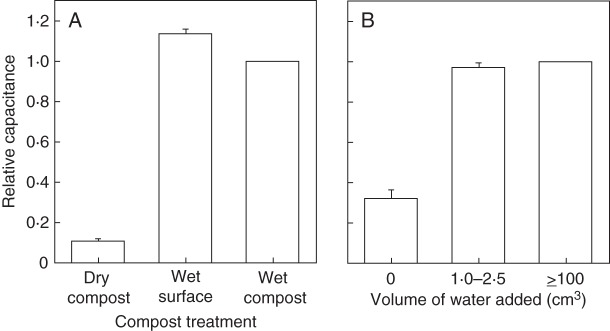
(A) Capacitance relative to fully wetted compost for barley plants in dry compost, compost wetted at the surface and thoroughly wetted compost. Data are expressed as mean ± s.e. (n = 35 plants). (B) Capacitance relative to fully wetted soil of tillers of barley plants growing in the field in 2010 to which zero, 1·0–2·5 or ≥100 cm3 of tap water was added around the stem base. Data are expressed as mean ± s.e. (n = 8 plants).
Fig. 5.

(A) Capacitance of barley plants growing in compost as the water table was increased (see Fig. 1) with values expressed relative to the final wetted capacitance. Data are means of 35 plants. Symbols represent individual plants. (B) Relationship between capacitance and the area of tissue in the stem cross-section of 20 barley plants measured with the water table at the compost surface. The linear regression, forced through the origin, is y = 1·3 ± 0·02x (R2 = 0·926). (C) Relationship between the shoot capacitances of 43 barley plants measured before and after their excision. The line indicates a 1:1 relationship. (We could identify no obvious reason for the single outlier plant in A.)
When the water table was at the compost surface (Fig. 1B), the capacitance of plants was linearly related to the area of tissue in the stem cross-section (Fig. 5B). This suggests that plant capacitance was determined by the dimensions of plant tissues close to the soil surface. When the shoot was excised, the capacitance of the tissue that had been between the compost surface and the original electrode inserted into the plant was almost identical to the capacitance measured for whole plants growing in compost (Fig. 5C). This implies a negligible contribution of roots (and also of the wet compost) to the observed capacitance.
Capacitances of plant and soil components combine in series
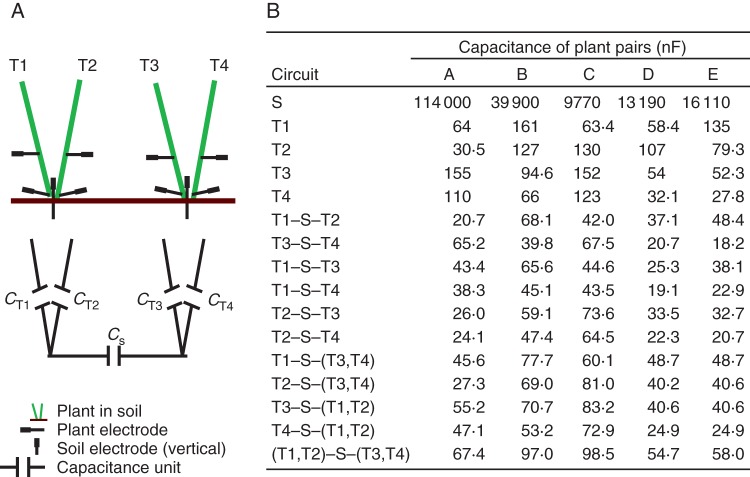
Dietrich et al. (2012) have proposed that capacitances of plant tissues and rooting substrate combine according to standard electrical circuit theory. This is a convenient (and powerful) simplification, but it must be remembered that this approach considers two-dimensional simplifications of real physical three-dimensional electric fields generated within heterogeneous root systems and their growth media. They consider plant tissue above the soil surface and the soil itself as individual components of the circuit. Their model predicts that the capacitance measured between two electrodes combines as the component capacitors wired in series, with capacitances of individual tillers of a plant attached to the same electrode acting in parallel (Fig. 6A). This model was tested on pairs of neighbouring plants in the field following rain. Data were collected for five pairs of neighbouring plants (Fig. 6B). The predicted capacitance (Cpred) was the capacitance estimated from an equation of the form:
| (1) |
where CT1 and CT2 were the capacitances of two tillers of one plant, CT3 and CT4 were the capacitances of two tillers of another plant, and CS was the capacitance of the soil between the steel electrodes situated at the bases of the two plants. There was good agreement between the measured capacitances and the capacitances predicted by Equation 1 (Fig. 7).
Fig. 6.
(A) Illustration showing the position of insertion of electrodes in tillers of neighbouring barley plants and in soil in August 2011, with the equivalent electrical circuit diagram below. (B) Capacitance measured between two electrodes in the soil (S), between two electrodes inserted at 3 mm and at 1·5 cm above the soil surface in an individual tiller (T1, T2, T3, T4), between electrodes attached at a height of 1·5 cm to individual tillers of the same plant (T1–S–T2, T3–S–T4) and between electrodes attached at a height of 1·5 cm to individual tillers of neighbouring plants (T1–S–T3, T1–S–T4, T2–S–T3, T2–S–T4) or to multiple combinations of tillers. Five pairs of neighbouring plants were studied (pairs A, B, C, D, E).
Fig. 7.

Relationship between the measured capacitance (Cm) and the capacitance predicted (Cp) using Equation 1 for the combination of tillers described in Fig. 6. The linear regression is Cp = 1·02 ± 0·008 Cm (R2 = 0·978, n = 54). Symbols represent data from different plant pairs.
GENERAL DISCUSSION
The capacitances of compost and soil increased with increasing water content (Fig. 2), as observed in previous studies (e.g. Robinson et al., 2005; Kizito et al., 2008; Wu et al., 2011).
When the substrates had water contents approaching field capacity, their capacitances were at least an order of magnitude greater than capacitances measured for plant tissue. Thus, the capacitance measured between an electrode in the rooting substrate and one inserted at the base of the stem would be dominated by plant tissue according to the model proposed by Dietrich et al. (2012). Wetting the substrate locally around the stem base was both necessary and sufficient to record a plant capacitance equal to that of the plant in fully wetted substrate (Figs 4 and 5A). This is consistent with the hypothesis that capacitance is dominated by tissue between the solution surface and the electrode attached to the plant, and that the bulk of the root system makes a negligible contribution to the measured capacitance (Dietrich et al., 2012). Indeed, when the shoot was excised, the capacitance of the tissue that had been between the compost surface and the original electrode inserted into the plant was almost identical to the capacitance measured for plants growing in compost (Fig. 5C). Capacitance was linearly related to the area of tissue in the stem cross-section when the water table was at the compost surface (Fig. 5B). This is similar to the findings of Dietrich et al. (2012) in hydroponics and suggests that the model proposed by Dietrich et al. (2012) could explain the variation in capacitance measurements made on cereals growing in solid substrates under various irrigation regimes. In addition, there was good agreement between the capacitances measured on pairs of neighbouring plants in the field and the capacitances predicted by Equation 1 (Fig. 7). This states explicitly that the capacitance measured between two electrodes combines as the component capacitors (plant tissue or solid substrate) wired in series, with capacitances of individual tillers of a plant connected to the same electrode acting in parallel.
Interestingly, our conclusions differ from recent findings of Ellis et al. (2012), who estimated the dielectric constant (relative permittivity) of root tissue as 69 assuming Dalton's cylindrical capacitance model for a bean root system grown in well-watered sand. Their estimated value is of similar magnitude to the dielectric constant for water and cellulose (80 and 7·6, respectively), and to other literature values mainly for woody or dried plant tissues (Ellis et al., 2013). This contrasts with a much greater apparent dielectric constant calculated from the stem data in Fig. 5B (dielectric constant of approx. 5 × 105; assuming the geometry of a parallel plate capacitor, with plate separation 3·3 mm – this is probably a slight underestimate of the apparent dielectric constant, as the stem was contacted by the electrodes, instead of using full parallel plates), that is of similar magnitude to apparent dielectric constants measured at 1 kHz reported elsewhere for other plant tissues (e.g. potato tuber tissue; Kulshrestha and Sastry, 2006, 2010; Wang et al., 2011) and some animal tissues (e.g. Gabriel et al., 1996). Further experimentation is necessary to clarify the dielectric properties of root and stem tissues in more detail, so that these contrasting results can be reconciled. As noted by Ellis et al. (2012), the dielectric constant will depend on tissue density effects, and additionally, for woody species, the four-terminal LCR method (Ellis et al., 2013) is likely to give improved readings of capacitance where the impedance at the electrode–tree interface is large.
CONCLUSIONS
All the findings presented herein are consistent with the model for plant capacitance developed by Dietrich et al. (2012), though these contrast with Ellis et al. (2012). Substrate capacitance and plant capacitance combine according to standard physical laws. Wetting the substrate locally around the stem base is both necessary and sufficient to record maximum capacitance. Under these conditions, plant tissue capacitance is much smaller than soil capacitance and, when these components are combined in series, the capacitance measured is largely determined by the tissue between the wet soil surface and the electrode attached to the plant. Whilst the measured capacitance might, in some circumstances, be correlated with root mass, it is not a direct measure of root mass.
ACKNOWLEDGEMENTS
R.C.D. was funded by a University of Dundee/James Hutton Institute PhD Studentship. The James Hutton Institute receives funding from the Scottish Government Rural and Environment Science and Analytical Services Division (RESAS). P.J.W. and A.G.B. gratefully acknowledge funding from the European Union (NUE Crops 222645 and EURoot 289300, respectively). We thank Dr Tim Ellis for his thorough helpful review of this manuscript.
LITERATURE CITED
- Cao Y, Repo T, Silvennoinen R, Lehto T, Pelkonen P. An appraisal of the electrical resistance method for assessing root surface area. Journal of Experimental Botany. 2010;61:2491–2497. doi: 10.1093/jxb/erq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Repo T, Silvennoinen R, Letho T, Pelkonen P. Analysis of the willow root system by electrical impedance spectroscopy. Journal of Experimental Botany. 2011;62:351–358. doi: 10.1093/jxb/erq276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chloupek O. Evaluation of the size of a plant's root system using its electrical capacitance. Plant and Soil. 1977;48:525–532. [Google Scholar]
- Dalton FN. In-situ root extent measurements by electrical capacitance methods. Plant and Soil. 1995;173:157–165. [Google Scholar]
- Dietrich RC, Bengough AG, Jones HG, White PJ. A new physical interpretation of plant root capacitance. Journal of Experimental Botany. 2012;63:6149–6159. doi: 10.1093/jxb/ers264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis T, Murray W, Kavalieris L. Electrical capacitance of bean (Vicia faba) root systems was related to tissue density – a test for the Dalton Model. Plant and Soil. 2012 http://dx.doi.org/10.1007/s11104-012-1424-z . [Google Scholar]
- Ellis T, Murray W, Keryn P, Kavalieris L, Brophy J, Williams C, Maass M. Electrical capacitance as a rapid and non-invasive indicator of root length. Tree Physiology. 2013;33:3–17. doi: 10.1093/treephys/tps115. [DOI] [PubMed] [Google Scholar]
- Gabriel C, Gabriel S, Corthout E. The dielectric properties of biological tissues. 1. Literature survey. Physics in Medicine and Biology. 1996;41:2231–2249. doi: 10.1088/0031-9155/41/11/001. [DOI] [PubMed] [Google Scholar]
- Kendall WA, Pederson GA, Hill RR., Jr Root size estimates of red clover and alfalfa based on electrical capacitance and root diameter measurements. Grass and Forage Science. 1982;37:253–256. [Google Scholar]
- Kizito F, Campbell CS, Campbell GS, et al. Frequency, electrical conductivity and temperature analysis of a low-cost capacitance soil moisture sensor. Journal of Hydrology. 2008;352:367–378. [Google Scholar]
- Kulshrestha SA, Sastry SK. Low-frequency dielectric changes in cellular food material from ohmic heating: effect of end point temperature. Innovative Food Science and Emerging Technologies. 2006;7:257–262. [Google Scholar]
- Kulshrestha SA, Sastry SK. Changes in permeability of moderate electric field (MEF) treated vegetable tissue over time. Innovative Food Science and Emerging Technologies. 2010;11:78–83. [Google Scholar]
- McBride R, Candido M, Ferguson J. Estimating root mass in maize genotypes using the electrical capacitance method. Archives of Agronomy and Soil Science. 2008;54:215–226. [Google Scholar]
- Ozier-Lafontaine H, Bajazet T. Analysis of root growth by impedance spectroscopy (EIS) Plant and Soil. 2005;277:299–313. [Google Scholar]
- Preston GM, McBride RA, Bryan J, Candido M. Estimating root mass in young hybrid poplar trees using the electrical capacitance method. Agroforestry Systems. 2004;60:305–309. [Google Scholar]
- Robinson DA, Kelleners TJ, Cooper JD, et al. Evaluation of a capacitance probe frequency response model accounting for bulk electrical conductivity: comparison with TDR and network analyzer measurements. Vadose Zone Journal. 2005;4:992–1003. [Google Scholar]
- Tsukahara K, Yamane K, Yamaki Y, Honjo H. A nondestructive method for estimating the root mass of young peach trees after root pruning using electrical capacitance measurements. Journal of Agricultural Meteorology. 2009;65:209–213. [Google Scholar]
- Urban J, Bequet R, Mainiero R. Assessing the applicability of the earth impedance method for in situ studies of tree root systems. Journal of Experimental Botany. 2011;62:1857–1869. doi: 10.1093/jxb/erq370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beem J, Smith ME, Zobel RW. Estimating root mass in maize using a portable capacitance meter. Agronomy Journal. 1998;90:566–570. [Google Scholar]
- Wang R, Zhang M, Mujumdar AS, Jiang H. Effect of salt and sucrose content on dielectric properties and microwave freeze drying behavior of re-structured potato slices. Journal of Food Engineering. 2011;106:290–297. [Google Scholar]
- Wu SY, Zhou QY, Wang G, Yang L, Ling CP. The relationship between electrical capacitance-based dielectric constant and soil water content. Environmental Earth Sciences. 2011;62:999–1011. [Google Scholar]