Abstract
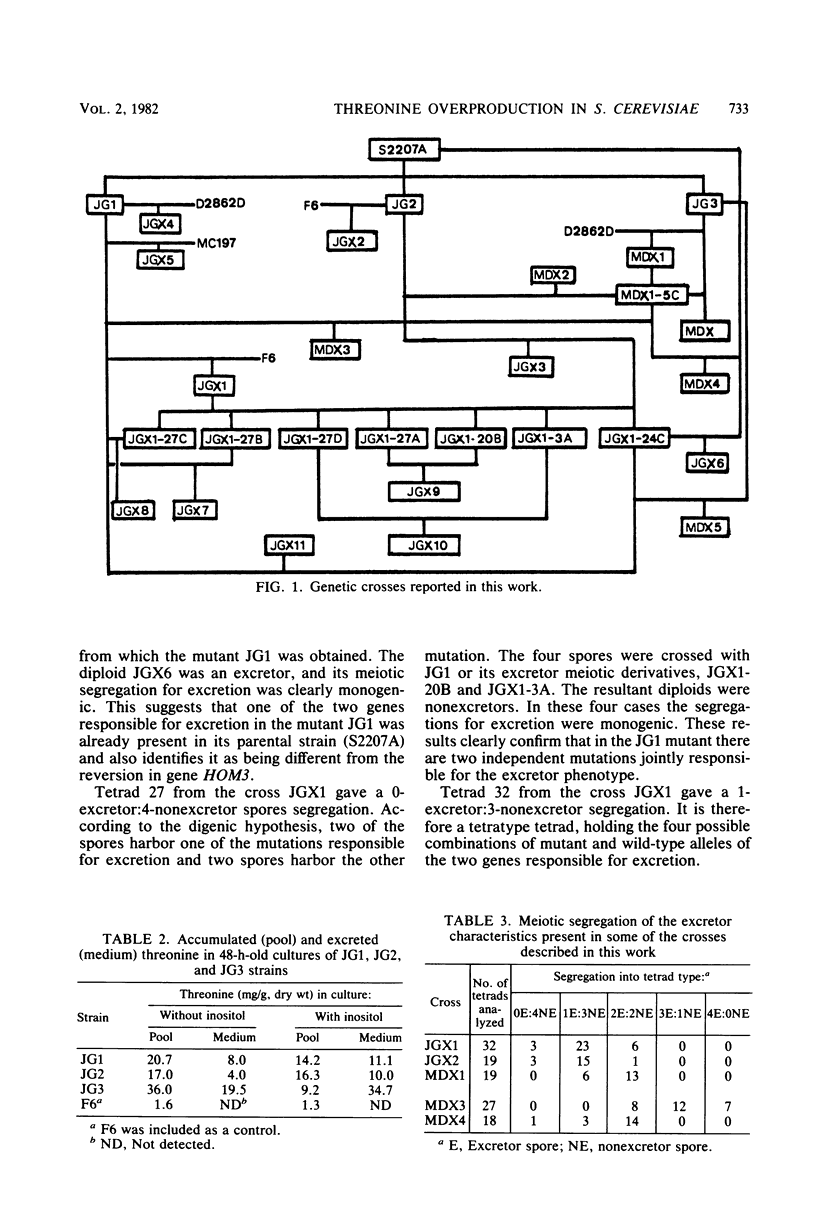
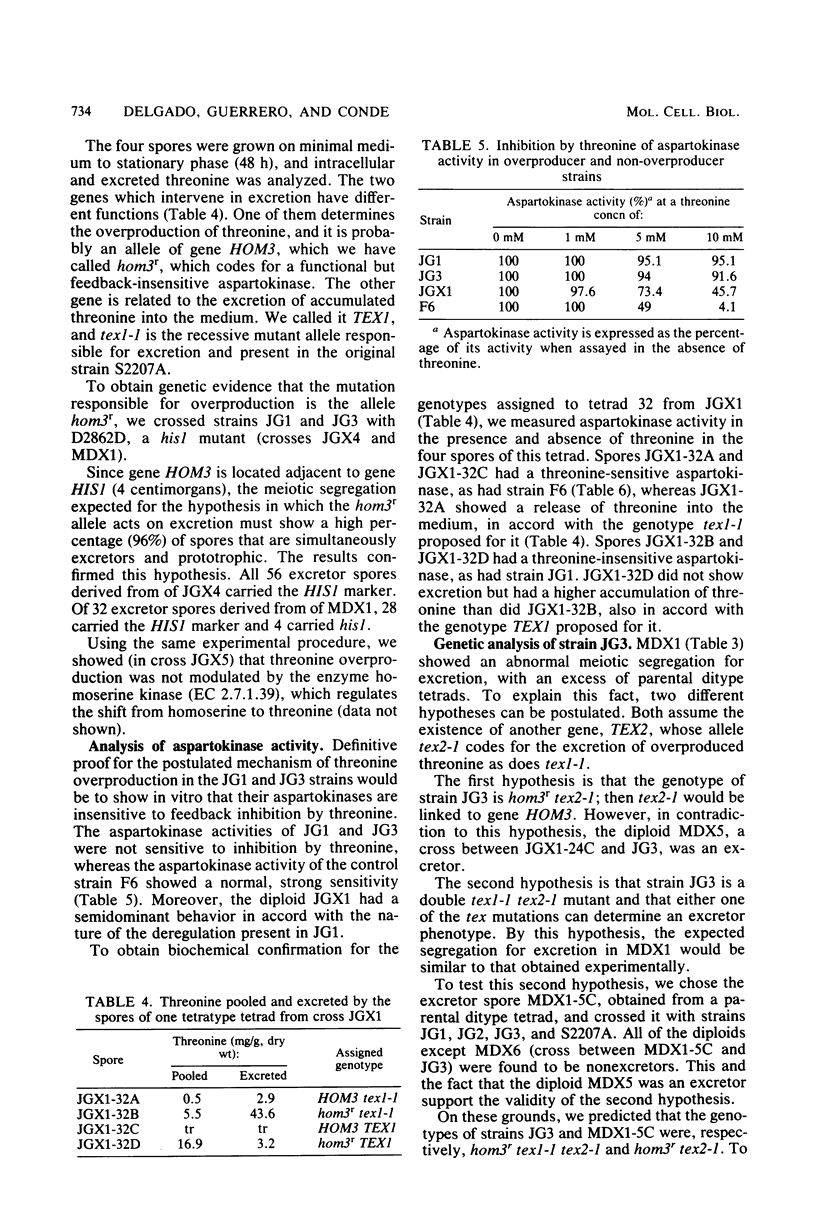
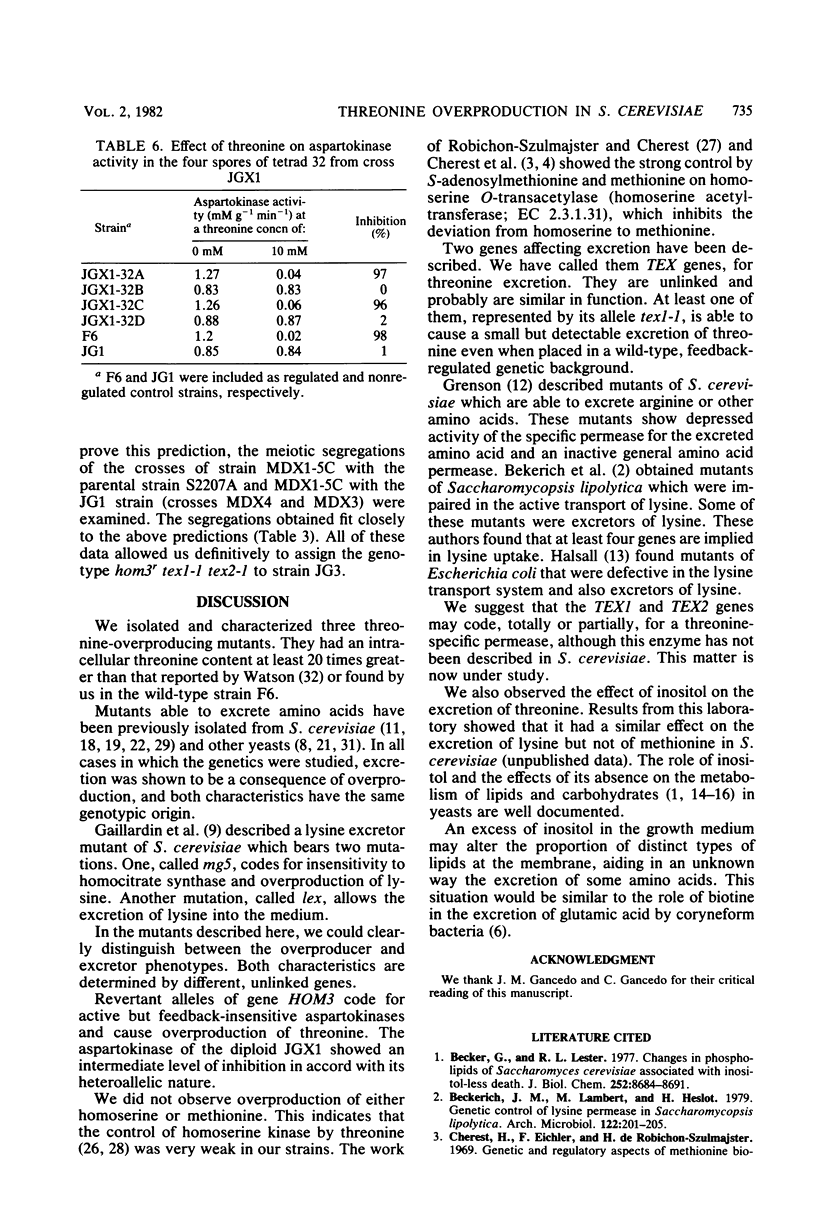
Three threonine-overproducing mutants were obtained as prototrophic revertants of a hom3 mutant strain of Saccharomyces cerevisiae. The gene HOM3 codes for aspartokinase (aspartate kinase; EC 2.7.2.4), the first enzyme of the threonine-methionine biosynthetic route, which is subjected to feedback inhibition by threonine. Enzymatic studies indicated that aspartokinase from the revertants has lost the feedback inhibition, resulting in overproduction of threonine. These revertants also bore one or two additional mutations, named tex1-1 and tex2-1, which alone or jointly made possible the excretion of the threonine accumulated. The effect of these two genes on excretion is potentiated by excess inositol in the medium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker G. W., Lester R. L. Changes in phospholipids of Saccharomyces cerevisiae associated with inositol-less death. J Biol Chem. 1977 Dec 10;252(23):8684–8691. [PubMed] [Google Scholar]
- Beckerich J. M., Lambert M., Heslot H. Genetic control of lysine permeases in Saccharomycopsis lipolytica. Arch Microbiol. 1979 Aug 6;122(2):201–205. doi: 10.1007/BF00411361. [DOI] [PubMed] [Google Scholar]
- Cherest H., Surdin-Kerjan Y., Robichon-Szulmajster H. Methionine-mediated repression in Saccharomyces cerevisiae: a pleiotropic regulatory system involving methionyl transfer ribonucleic acid and the product of gene eth2. J Bacteriol. 1971 Jun;106(3):758–772. doi: 10.1128/jb.106.3.758-772.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robichon-Szulmajster Régulation du fonctionnement de deux chaines de biosynthèse chez saccharomyces cerevisiae: thréonine-méthionine et isoleucine-valine. Bull Soc Chim Biol (Paris) 1967 Dec 18;49(11):1431–1462. [PubMed] [Google Scholar]
- Demain A. L., Birnbaum J. Alteration of permeability for the release of metabolites from the microbial cell. Curr Top Microbiol Immunol. 1968;46:1–25. doi: 10.1007/978-3-642-46121-7_1. [DOI] [PubMed] [Google Scholar]
- Demain A. L., Jackson M., Vitali R. A., Hendlin D., Jacob T. A. Production of guanosine-5'-monophosphate and inosine-5'-monophosphate by fermentation. Appl Microbiol. 1966 Sep;14(5):821–825. doi: 10.1128/am.14.5.821-825.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillardin C. M., Sylvestre G., Heslot H. Studies on an unstable phenotype induced by UV irradiation: the lysine excreting (lex(-)) phenotype of the yeast Saccharomycosis lipolytica. Arch Microbiol. 1975 Jun 20;104(1):89–94. doi: 10.1007/BF00447305. [DOI] [PubMed] [Google Scholar]
- Gaillardin C., Heslot H. Evidence for mutations in the structural gene for homocitrate synthase in Saccharomycopsis lipolytica. Mol Gen Genet. 1979 May 4;172(2):185–192. doi: 10.1007/BF00268281. [DOI] [PubMed] [Google Scholar]
- Gehrke C. W., Zumwalt R. W., Kuo K. Quantitative amino acid analysis by gas-liquid chromatography. J Agric Food Chem. 1971 Jul-Aug;19(4):605–618. doi: 10.1021/jf60176a006. [DOI] [PubMed] [Google Scholar]
- Gray G. S., Bhattacharjee J. K. Biosynthesis of lysine in Saccharomyces cerevisiae: regulation of homocitrate synthase in analogue-resistant mutants. J Gen Microbiol. 1976 Nov;97(1):117–120. doi: 10.1099/00221287-97-1-117. [DOI] [PubMed] [Google Scholar]
- Halsall D. M. Overproduction of lysine by mutant strains of Escherichia coli with defective lysine transport systems. Biochem Genet. 1975 Feb;13(1-2):109–124. doi: 10.1007/BF00486010. [DOI] [PubMed] [Google Scholar]
- Hanson B. A., Lester R. L. Effects of inositol starvation on phospholipid and glycan syntheses in Saccharomyces cerevisiae. J Bacteriol. 1980 Apr;142(1):79–89. doi: 10.1128/jb.142.1.79-89.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi E., Hasegawa R., Tomita T. Accumulation of neutral lipids in Saccharomyces carlsbergensis by myo-inositol deficiency and its mechanism. Reciprocal regulation of yeast acetyl-CoA carboxylase by fructose bisphosphate and citrate. J Biol Chem. 1976 Sep 25;251(18):5759–5769. [PubMed] [Google Scholar]
- Henry S. A., Atkinson K. D., Kolat A. I., Culbertson M. R. Growth and metabolism of inositol-starved Saccharomyces cerevisiae. J Bacteriol. 1977 Apr;130(1):472–484. doi: 10.1128/jb.130.1.472-484.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Masselot M., de Robichon-Szulmajster H. Methionine biosynthesis in Saccharomyces cerevisiae: mutations at the regulatory locus ETH2. I. Genetic data. Mol Gen Genet. 1974 Apr 3;129(4):339–348. doi: 10.1007/BF00265697. [DOI] [PubMed] [Google Scholar]
- Masselot M., de Robichon-Szulmajster H. Methionine biosynthesis in Saccharomyces cerevisiae: mutations at the regulatory locus ETH2. II. Physiological and biochemical data. Mol Gen Genet. 1974 Apr 3;129(4):349–361. doi: 10.1007/BF00265698. [DOI] [PubMed] [Google Scholar]
- Morzycka E., Sawnor-Korszyńska D., Paszewski A., Grabski J., Raczyńska-Bojanowska K. Methionine overproduction by Saccharomycopsis lipolytica. Appl Environ Microbiol. 1976 Jul;32(1):125–130. doi: 10.1128/aem.32.1.125-130.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh M., Schlegel H. G. Die Biosynthese von Isoleucin und Valin in Hydrogenomonas H 16. Arch Mikrobiol. 1969;67(2):110–127. [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Cherest H. Regulation of homoserine O-transacetylase, first step in methionine biosyntheis in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1967 Jul 21;28(2):256–262. doi: 10.1016/0006-291x(67)90438-x. [DOI] [PubMed] [Google Scholar]
- Schürch A., Miozzari J., Hütter R. Regulation of tryptophan biosynthesis in Saccharomyces cerevisiae: mode of action of 5-methyl-tryptophan and 5-methyl-tryptophan-sensitive mutants. J Bacteriol. 1974 Mar;117(3):1131–1140. doi: 10.1128/jb.117.3.1131-1140.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson T. G. Amino-acid pool composition of Saccharomyces cerevisiae as a function of growth rate and amino-acid nitrogen source. J Gen Microbiol. 1976 Oct;96(2):263–268. doi: 10.1099/00221287-96-2-263. [DOI] [PubMed] [Google Scholar]


