Figure 10.
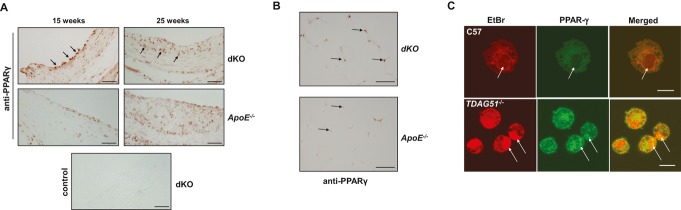
TDAG51 deficiency increases PPARγ expression and nuclear localization in lesion‐resident macrophages. A, TDAG51−/−/ApoE−/− (dKO) or ApoE−/− mice were placed on control chow diet for 15 or 25 weeks. Atherosclerotic lesions from the aortic roots were sectioned and immunostained for PPARγ. Arrows indicate PPARγ‐positive staining macrophages. Representative images from 5 mice per group are shown. Scale bar=50 μm. B, Identification of PPARγ in adipose tissue from TDAG51+/+/ApoE−/− (ApoE−/−) and TDAG51−/−/ApoE−/− (dKO) mice fed chow diet for 40 weeks. Fat pads were removed, embedded in paraffin, sectioned, and immunostained for PPARγ. Arrows indicate positive nuclear immunostaining for PPARγ. Consistent with lesion‐resident TDAG51−/− macrophages, intensity of nuclear PPARγ staining was increased in TDAG51−/− adipocytes. Representative images from 5 mice per group are shown. Scale bar=100 μm. C, Optical sections 0.8 μm in thickness were obtained through TDAG51−/− and C57BL/6 (C57) peritoneal macrophages at the plane of the nuclei (arrows). In C57 macrophages, little PPARγ (green) was visualized within the nucleus, whereas in TDAG51−/− macrophages, PPARγ (green) was found to colocalize (merged green and red producing yellow) with nucleic acids (red) as shown by ethidium bromide (EtBr) staining in the nuclei (arrows). Representative images from 3 independent experiments are shown. Scale bar=10 μm. TDAG51 indicates T‐cell death‐associated gene 51; ApoE, apolipoprotein E; dKO, double knockout; PPARγ, peroxisome proliferator‐activated receptor γ.
