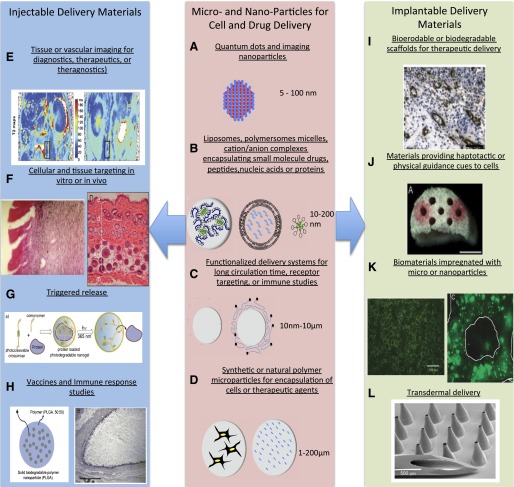
Fig. 5.
Methods to generate functionalized biomaterials for regenerative medicine. Micro- and nanoparticles for cell and drug delivery (center): several micro- and nanoparticle systems are highlighted schematically. (A) Nanoparticles used for imaging modalities include quantum dots (fluorescence) and iron oxide nanoparticles (magnetic resonance imaging). Nanoparticles with hollow centers are can also be loaded with iodine or other image contrast agents. A schematic of the structure of a quantum dot nanoparticle is shown. (B) In addition to contrast agents, small molecule drugs, nucleic acids, peptides, and protein drugs can be loaded into a variety of self-assembling nanoparticle systems that typically range from 10 to 200 nm. Schematics of DNA-polymer complexes, liposomes, and micelles are shown. (C) These nanoparticles can be surface modified with polyethylene glycol (left) to improve pharmacokinetics or can be modified with targeting motifs to improve cellular uptake (right). (D) Larger microscale constructs can also be formed from natural and synthetic polymers for release of therapeutic agents (right) or the delivery of cells (left) to provide cell-based delivery of, for example, insulin in the treatment of diabetes (Opara et al., 2010). Injectable delivery materials (left): the delivery systems described in the center panel have multiple applications to regenerative medicine when delivered either systemically or locally. (E) Shown is the in vivo tracking of implanted scaffolds containing cells loaded with ultrasmall superparamagnetic iron oxide nanoparticles (Reprinted with permission from Harrington et al., 2011). (F) The use of cationic liposomes to deliver DNA encoding for IGF-1 (and Lac-Z for imaging purposes) is shown at left (Reproduced with permission of BENTHAM SCIENCE PUBLISHERS LTD; Jeschke MG, Herndon DN, Baer W, Barrow RE, and Jauch KW (2001) Possibilities of non-viral gene transfer to improve cutaneous wound healing. Curr Gene Ther 1:267–278), whereas the delivery and protection of Wnt proteins for control of hair follicle stem cells to promote dermal thickening and follicle neogenesis in mice is shown at right (Morrell et al., 2008). (G) The ability to not only localize drugs but have the release of their payload triggered by internal [e.g., pH, temperature change, enzymes) or external (temperature, ultrasound, or as shown, light sources (Reprinted with permission from Azagarsamy MA, Alge DL, Radhakrishnan SJ, Tibbitt MW, and Anseth KS (2012) Photocontrolled nanoparticles for on-demand release of proteins. Biomacromolecules 13:2219–2224. Copyright © 2012, American Chemical Society)]. (H) Incorporation of antigens into microparticles or nanoparticles for improved vaccine delivery is shown at left (Reprinted with permission from Demento SL, Cui W, Criscione JM, Stern E, Tulipan J, Kaech SM, and Fahmy TM (2012) Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials 33:4957–4964) while the use of biomaterial implants is aiding in elucidating and ultimately minimizing inflammatory responses to implanted materials (Reprinted with permission from Norton LW, Park J, and Babensee JE (2010) Biomaterial adjuvant effect is attenuated by anti-inflammatory drug delivery or material selection. J Control Release 146:341–348). Implantable delivery materials (right), delivery systems shown in the center panel may or may not be part of implantable biomaterial scaffolds as well. (I) One example of this achieving spatiotemporal control over multiple growth factors in which one factor is released rapidly from the scaffold material (e.g., VEGF) and a second growth factor (e.g., platelet-derived growth factor) is released at a slower rate from embedded microparticles to promote angiogenesis or support other aspects of tissue formation (Reprinted with permission from Chen RR, Silva EA, Yuen WW, and Mooney DJ (2007) Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res 24:258–264). (J) Materials that contain specific topography or pore architecture to simulate native tissue in an increasingly important concept in biomaterial design. As shown, conduits to promote nerve regeneration are advantageous, and the delivery of nerve growth factor from microparticles or the incorporation of extracellular matrix cues such as fibronectin supports these processes (Reprinted with permission from De Laporte L, Huang A, Ducommun MM, Zelivyanska ML, Aviles MO, Adler AF, and Shea LD (2010) Patterned transgene expression in multiple-channel bridges after spinal cord injury. Acta Biomater 6:2889–2897). (K) Methods to incorporate microparticles or nanoparticles into biomaterial scaffolds include incorporation into the matrix of the scaffold (Reprinted with permission from Lee M, Chen TT, Iruela-Arispe ML, Wu BM, and Dunn JC (2007) Modulation of protein delivery from modular polymer scaffolds. Biomaterials 28:1862–1870) or coating onto the scaffold’s pores (Reprinted with permission from Saul JM, Linnes MP, Ratner BD, Giachelli CM, and Pun SH (2007) Delivery of non-viral gene carriers from sphere-templated fibrin scaffolds for sustained transgene expression. Biomaterials 28:4705–4716). (L) Another materials-based approach important to the delivery of therapeutics related to regenerative medicine are microneedle patches that overcome diffusion barriers in the skin to allow more efficient, long-term delivery of therapeutics (Reprinted with permission from Davis SP, Martanto W, Allen MG, and Prausnitz MR (2005) Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng 52:909–915).