Fig. 9.
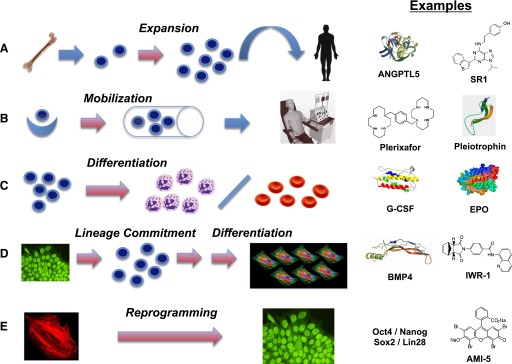
Five strategies by which regenerative pharmacology can affect biology of stem and progenitor cells. Red arrows indicate steps at which a pharmacological agent may be applied. (A) Expansion of lineage-restricted stem or progenitor cells (depicted in cartoon form as blue cells with large nuclei). Exemplified here by isolation of hematopoietic stem cells (HSC, e.g., CD34-positive cells) from human bone marrow and expansion in culture using a cocktail of cytokines/growth factors including angiopoietin-like 5 (AGPTL5) (Drake et al., 2011), prior to infusion into a patient small molecules, such as an aryl hydrocarbon receptor antagonist designated StemRegenin 1 (SR1) (Boitano et al., 2010), likewise can promote HSC expansion in culture. (B) Mobilization of stem cells from an endogenous niche. Exemplified by recruitment of stem cells from tissue niches (blue crescent) to enter the circulation (cylinder depicts a blood vessel) and potentially to undergo expansion prior to further lineage commitment. Plerixafor (Brave et al., 2010) is a small molecule that drives HSC mobilization from bone marrow niches, used in combination with G-CSF. Pleiotrophin (Himburg et al., 2010; Istvanffy et al., 2011) promotes expansion of the HSC pool in vivo. The cells are collected from a donor by apheresis. (C) Differentiation of committed progenitor cells to functional, specialized cells. Exemplified by accelerated maturation of granulocyte progenitors to infection-fighting neutrophils (left) promoted by G-CSF (Frampton et al., 1994) and of erythroid progenitors to red blood cells (right) promoted by erythropoietin (EPO) (Faulds and Sorkin, 1989). (D) Production of specialized cells from pluripotent stem cells (ES or iPS cells) by sequential steps of lineage commitment and terminal differentiation. Pluripotent stem cells are depicted iconically as a cluster with nuclei stained for the pluripotency-associated transcription factor Oct4. Cardiomyocytes (shown iconically by staining for cardiac troponin and other heart-specific proteins) represent a cell type that might be used in tissue engineering and for drug discovery. A key factor in the promotion of commitment to mesodermal and cardiac fates is BMP-4 (Evseenko et al., 2010; Hogan, 1996; Kattman et al., 2011; Murry and Keller, 2008). Small molecule inhibitors of Wnt/beta-catenin signaling, such as IWR-1 (Chen et al., 2009; Willems et al., 2011), drive the generation of cardiomyocytes from human ES cell-derived mesoderm. (E) Production of genetically compatible induced pluripotent stem (iPS) cells from an individual’s own cells. Autologous cells such as skin fibroblasts (shown iconically by staining for F-actin in the cytoskeleton) are reprogrammed to pluripotency by exposure to a set of four transcription factors [e.g., the four identified by the Thomson group—Oct4, Nanog, Sox2, and Lin 28 (Yu et al., 2007)]. The small molecule AMI-5 (Yuan et al., 2011b), an inhibitor of protein arginine methyltransferase (PRMT), enables reprogramming in conjunction with Oct4 alone.
