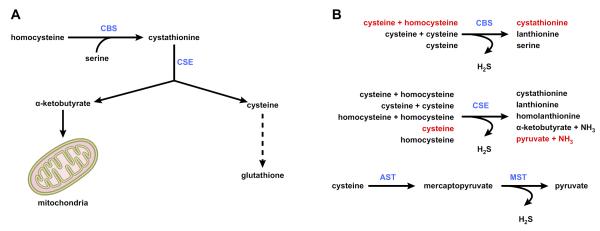
FIGURE 3.
Methionine metabolism: transsulfuration and hydrogen sulfide synthesis. A: the transsulfuration pathway links AdoMet to cysteine biosynthesis. Here homocysteine is converted to cysteine (the rate-limiting precursor for glutathione) via a two-step enzymatic process catalyzed by cystathionine β-synthase (CBS) and cystathionase (CSE), both requiring vitamin B6. α-Ketobutyrate, the other product of cystathionine cleavage, is further metabolized by the mitochondria through the Kreb's cycle. B: hydrogen sulfide synthesis. Although the classical role of CBS and CSE is to generate cystathionine and cysteine, respectively, these two enzymes catalyze multiple hydrogen sulfide (H2S)-generating reactions using cysteine and homocysteine as substrates. The predominant H2S-generating reactions are shown in red. A third pathway uses aspartate aminotransferase (AST) to yield mercaptopyruvate, which is further converted into H2S in a reaction catalyzed by mercaptopyruvate sulfurtransferase (MST). The function of this pathway is primarily catabolic.