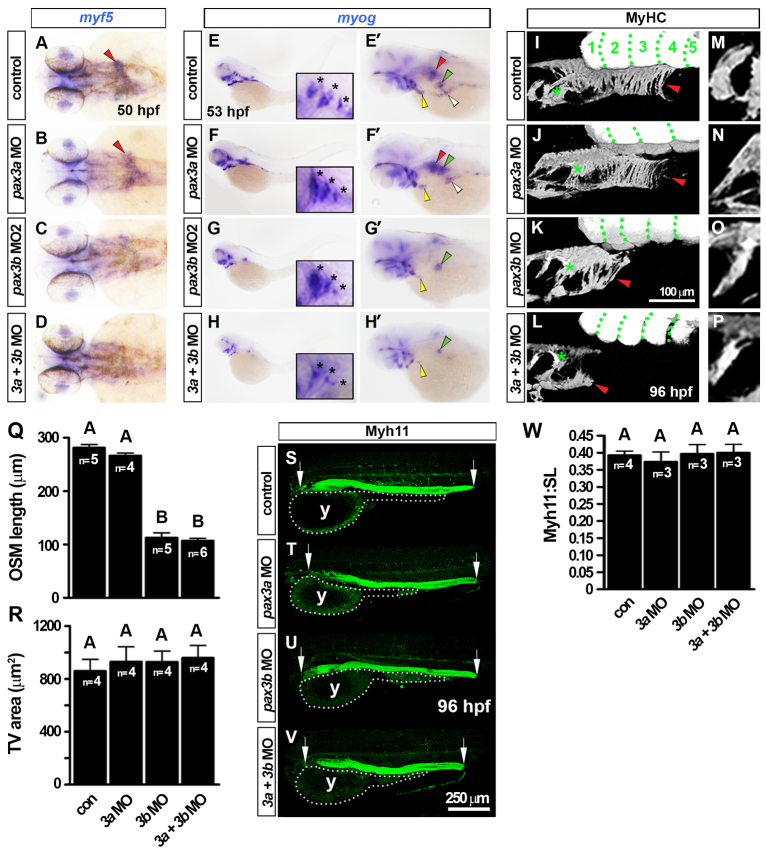
Fig. 6.
pax3b morphants have reduced OSM but unaffected development of gastrointestinal smooth muscle. In situ hybridisation for myf5 (A-D) and myog (E-H′) and immunodetection of MyHC (I-P) and Myh11 (S-V). Wholemounts have anterior towards the left in dorsal (A-D), lateral (E-H, I-P,S-V; dorsal to top) or dorsal oblique views (E′-H′). (A-D) Loss of myf5 mRNA from the ‘inverse fountain’ OSM region in pax3b morphants (red arrowheads). (E-H′) Reduction in myog mRNA in pax3b morphant OSM (red arrowheads), SHM (yellow arrowheads), PHM (white arrowheads) and PFM (green arrowheads). In pax3a morphants, myog expression in all muscles was indistinguishable from controls. Insets and asterisks show comparable opercular muscle developmental in pax3b morphants and controls. (I-L) At 96 hpf, MyHC had accumulated in control and pax3a morphant OSM (red arrowheads), extending caudally to somite 3-4. Green dots delineate somites. pax3b and pax3b+pax3a morphants had severely reduced OSM (red arrowheads). (M-Q) Shape, orientation and area of tranversus ventralis (TV) muscles were unaffected after pax3 manipulation. (R) OSM length was measured from the caudal-most hypobranchial muscle in the pharynx (asterisks in I-L) to the caudal edge of OSM (red arrowheads in I-L). A and B groups differ significantly (P<0.0001). (S-V) Myh11 was unchanged after pax3 manipulation. Arrows indicate anterior and posterior extent for Myh11 length measurements. (W) Fraction of larvae standard length (SL) occupied by Myh11+ was not altered after pax3 manipulation. y, yolk. Data are mean+s.e.m.