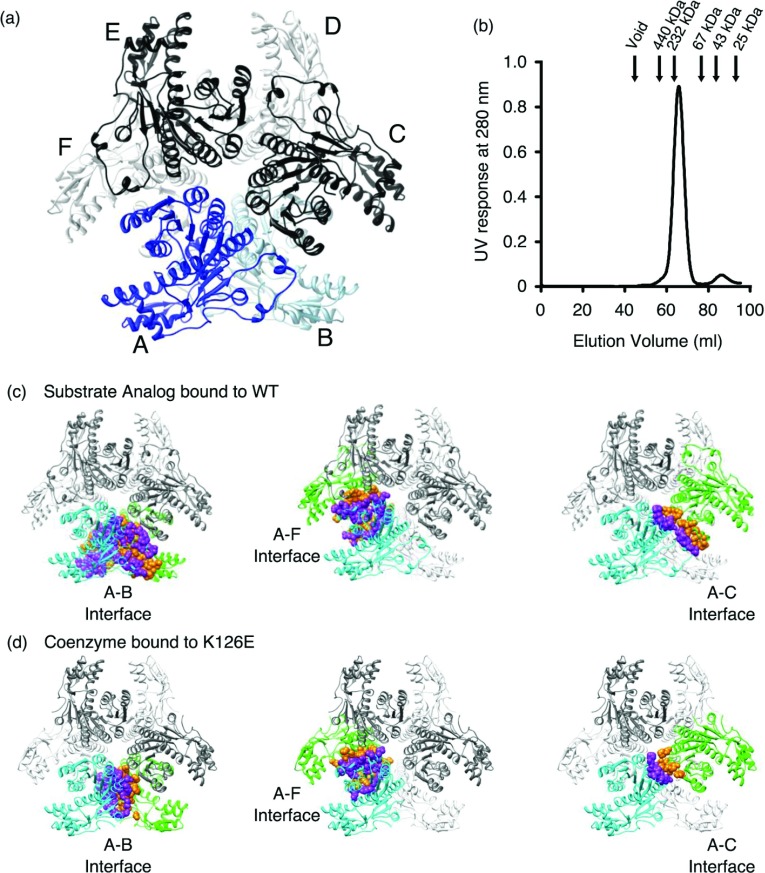
Figure 4. Hexameric organization of CapE.
(a) CapE forms a hexamer organized as a trimer of dimers (3×2). The structure corresponds to wild-type CapE in complex with substrate analogue (ligand is not shown). The protein subunits of one of the pseudo-dimers are depicted in dark and light blue (chains A and B). The other subunits are depicted in black and grey. (b) SEC profile of CapE. The arrows indicate the elution of the calibration standards. CapE elutes with an apparent molecular weight of 210 kDa. A minor fraction of monomer (8%) with an apparent molecular weight of 40 kDa is also observed. The predicted molecular weight of one subunit of CapE is 39 kDa, and that of the hexamer is 230 kDa. The peak corresponding to the dimer was not observed. (c) Interaction surfaces between protein subunits. The three main contact interfaces correspond to subunits A–B, A–F and A–C. (d) Same interaction surfaces in mutein K126E.