Figure 1.
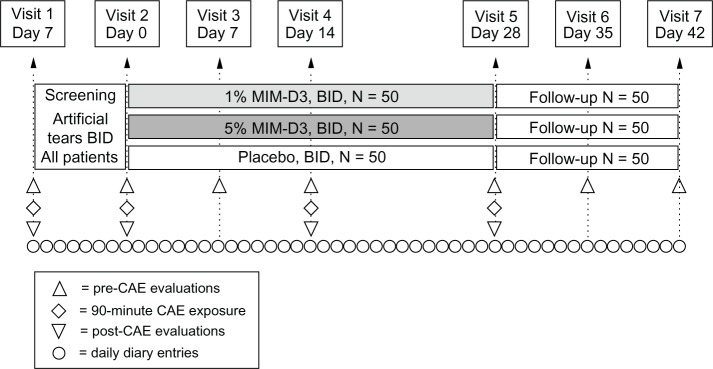
Visit flow chart of a two-center, randomized, double-masked, placebo-controlled study with a novel TrkA agonist (MIM-D3) in patients with a history of dry eye and objective evidence of ongoing dry eye disease.
Notes: The study, which included a 1-week run-in period with artificial tears, comprised two visits of screening/eligibility for patients with modifiable signs and symptoms. randomization and first dose on-site at visit 2 (day 0) were followed with three visits during the treatment period, for endpoint and safety measurements, and two visits of post-treatment follow up for efficacy and safety.
Abbreviations: BID, twice daily; TrkA, receptor tyrosine kinase-A; CAE, controlled Adverse environment.
