Figure 5.
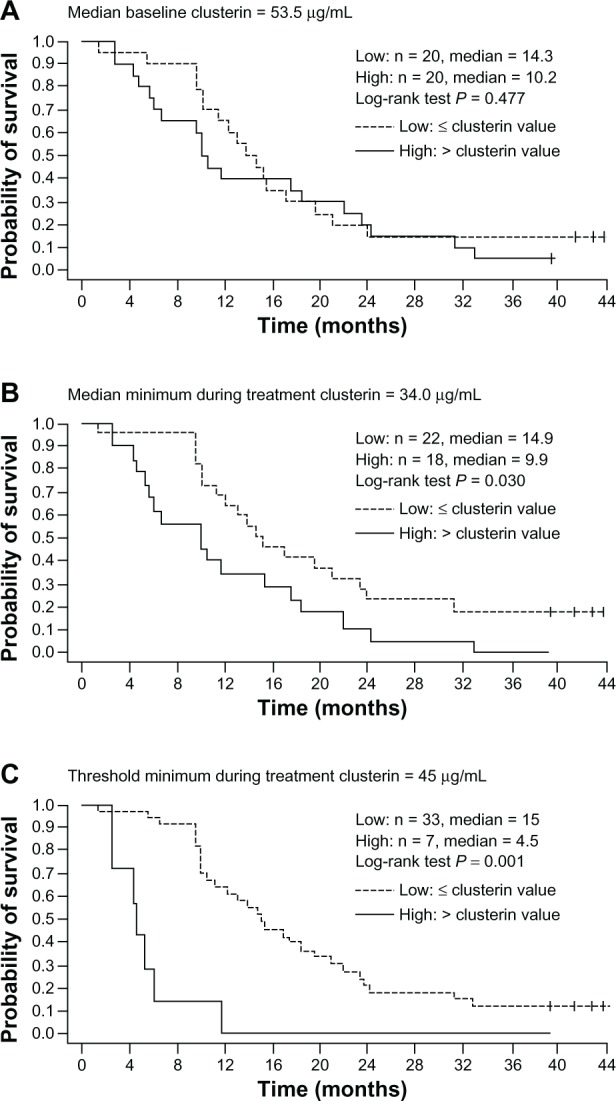
Relationship between overall survival and serum clusterin levels at baseline and during custirsen treatment, based on Kaplan-Meier estimates for dichotomous classifications of patients. (A) Median baseline clusterin (≤ median versus > median), (B) median minimum clusterin during treatment (≤ median versus > median); and (C) threshold minimum during treatment with 45 μg/mL (≤45 μg/mL versus >45 μg/mL).68
Note: Reprinted from Clinical Cancer Research 2011, volume 17, pages 5765–5773. Saad F, Hotte S, North S, Eigl B, Chi K, Czaykowski P, Wood L, Pollak M, Berry S, Lattouf JB, Mukherjee SD, Gleave M, Winquist E; Canadian Uro-Oncology Group, Randomized phase II trial of custirsen (OGX-011) in combination with docetaxel or mitoxantrone as second-line therapy in patients with metastatic castrate-resistant prostate cancer progressing after first-line docetaxel: CUOG trial P-06c, with permission from AACR.68
