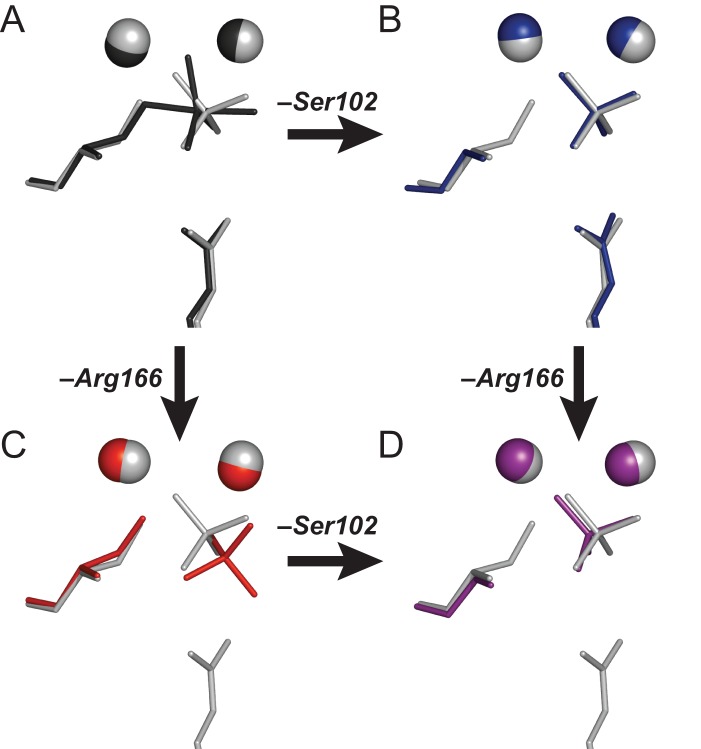
Figure 3. Structural comparisons of noncovalently bound Pi in AP and Ser102 mutants.
(A) Overlay of WT AP with vanadate transition state analog covalently bound to Ser102 (black, 1PDB code 1B8J) and Pi noncovalently bound (gray, PDB code 3TGO). (B) Overlay of WT AP (grey) and S102G AP (blue, PDB code 1ELZ), both with bound Pi. (C) Overlay of WT AP (grey) and R166S AP (red, PDB code 3CMR). Mutation of Arg166 to Ser results in rotation and 1.0 Å translation of the bound Pi. (D) Overlay of WT AP (grey) and S102G/R166S AP (purple). Removal of the Arg166 side chain (R166S AP) results in a rearrangement of the bound Pi with Ser102 present (A→C) but not with Ser102 mutated (B→D) (see Text S5). While it is likely, based on the results herein and previously [16], that Pi is bound as the trianion in all cases and Ser102 is protonated when present, the X-ray data lack the resolution needed to identify protons.