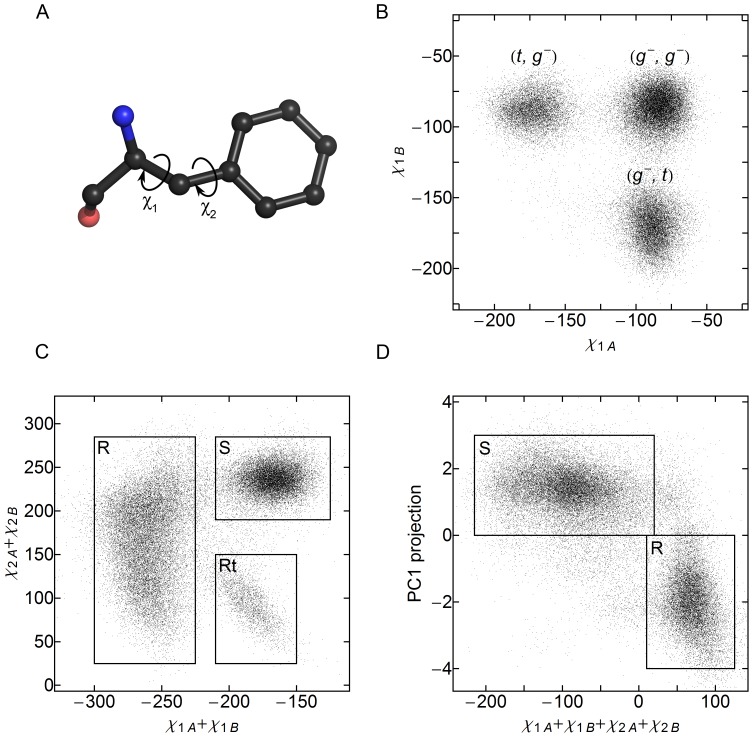
Figure 6. Use of F124 χ1 and χ2 dihedral angles to classify the HAMP domain state.
Subscripts A and B denote the protomers of the dimeric HAMP domain. (A) Definition of the χ1 and χ2 dihedral angles of phenylalanine sidechain. (B) Distribution of the χ1 angles during the simulations. t stands for the trans conformation, and g- for the gauche- conformation. (t, t) conformation is not observed. (C) Plot of the sum of the χ2 angles as a function of the sum of the χ1 angles. There are two substates corresponding to χ1 (g-, g-) conformation (χ1 sum of ∼-170°). The state with the χ2 sum of ∼240° corresponds to the signaling state, meanwhile the state with the sum of ∼100° is transient and visited during transitions from the χ1 (t, g-) conformation to (g-, t). (D) Relation between the F124 conformations, represented by the sum of side chain dihedral angles, and the whole domain backbone conformation, represented by the value of the projection on the first principal component. Unambiguous correspondence may be established between these two values.