Figure 5.
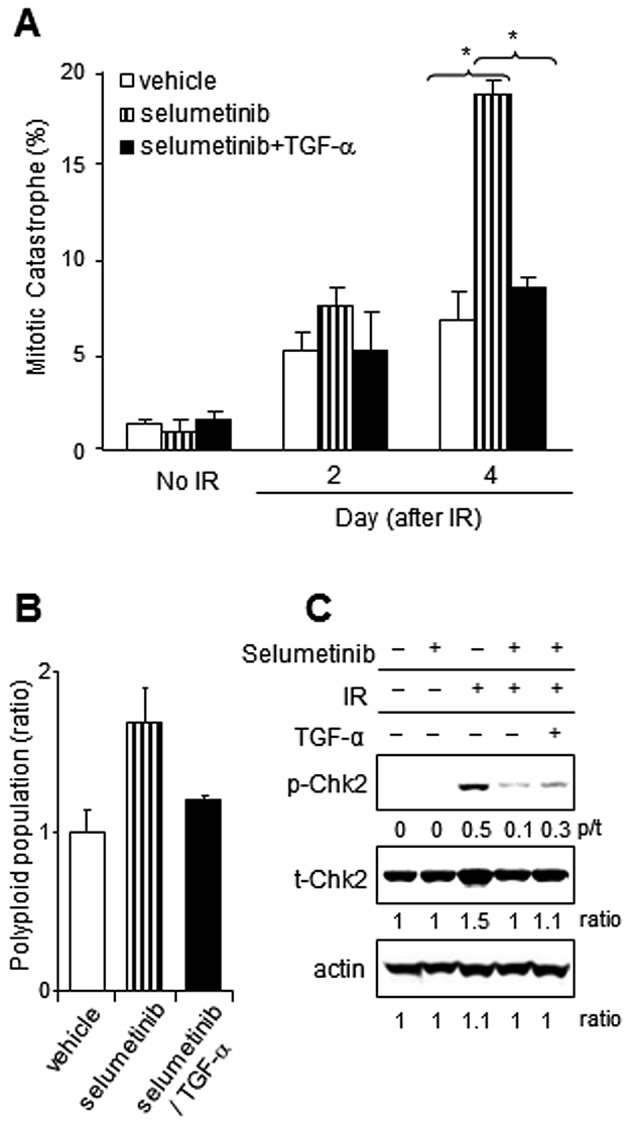
Effects of TGF-α on enhanced mitotic catastrophe induced by selumetinib after radiation in A549. (A) Mitotic catastrophe: Cells growing in chamber slides were exposed to selumetinib (250 nM) or the vehicle control, IR (4 Gy), with or without the addition of TGF-α and fixed at the specified times for immunocytochemical analysis for mitotic catastrophe. Nuclear fragmentation was evaluated in 150 cells per treatment from 5 different fields. Nuclear fragmentation was defined as the presence of ≥2 distinct lobes within a single cell. TGF-α supplementation reduced mitotic catastrophe in A549 cells treated with selumetinib and IR. Columns, mean; bars, SE. Nuclear fragmentation was defined as the presence of ≥2 distinct lobes within a single cell. *P<0.05 according to the Student’s t-test (selumetinib vs. selumetinib + TGF-α). (B) Polyploid population: Polyploid cells containing abnormal DNA (>4 n) were detected by flow cytometry in A549 cells treated as indicated at 24 h after IR exposure. Polyploidy after IR exposure was enhanced by selumetinib, however TGF-α addition reduced the level of polyplod population down to the level of IR alone. (C) Western blot analysis for phosphorylated Chk2: Chk2 is known a regulator of mitotic catastrophe. The level of activated Chk2 was investigated by immunoblotting in A549 cells treated with selumetinib (250 nM), IR (4 Gy) and rhTGF-α (10 pg/ml) 24 h following IR.
