Figure 2.
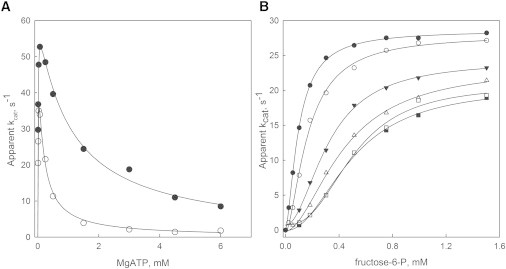
Effect of KCl on the allosteric inhibition of Pfk-2 induced by MgATP. (A) Initial velocity of Pfk-2 measured as a function of MgATP at 50 μM fructose-6-P in the presence (o) or absence (•) of 50 mM KCl. Continuous lines represent the fit to a model of uncompetitive substrate inhibition. Kinetic constants are shown in Table 1. (B) Initial velocities of Pfk-2 obtained with fructose-6-P as a variable substrate under inhibitory MgATP conditions (5 mM) at different fixed KCl concentrations of 0 (•), 7.5 (o), 17 (▾), 50 (▵), 75 (□), and 150 mM (■). Continuous lines represent the individual fit of each curve to the Hill equation.
