Figure 5.
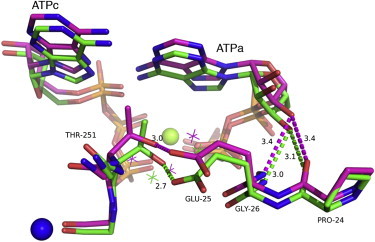
Structural superposition of the monovalent cation site in the presence and absence of Cs+. The backbone of the monovalent cation site is represented in green (Pfk-2-MgATP complex; PDBid 3CQD, chain B) and magenta sticks (Pfk-2-MgATP-Cs complex PDBid 3UMP). The green sphere corresponds to Mg2+ coordinated by the phosphates of the ATPc and ATPa. Upon binding of Cs+ (blue sphere), Thr-251 drawn the side chain of Glu-25 resulting in increments of the hydrogen bonds distances between the backbone groups of Pro-24 and Gly-26 and the O3′ oxygen of the ribose of the allosteric ATP from 3.1 to 3.4 Å on average. This change is also accompanied by rearrangements of the water network observed in the presence (magenta asterisks) and absence of Cs+ (green asterisks).
