Figure 6.
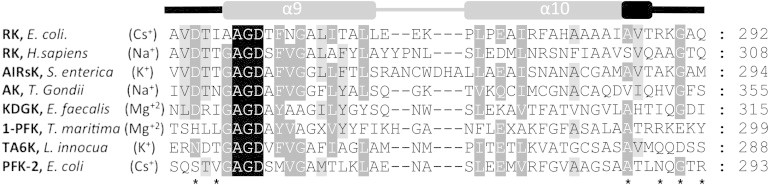
Multiple sequence alignment of the region forming the cation-binding site in members of the ribokinase family. The structures were obtained from the Protein Data Bank as follows: ribokinases (RK) from Escherichia coli (PDBid 1GQT) and Homo sapiens (PDBid 2FV7), aminoimidazole riboside kinase (AIRsK) from Salmonella enterica (PDBid 1TZ6), adenosine kinase (AK) from Toxoplasma gondii (PDBid 2ABS), putative 2-keto-3-deoxygluconate kinase (KDGK) from Enterococcus faecalis (PDBid 3KTN), putative 1-phosphofructokinase (1-PFK) from Termotoga maritima (PDBid 2AJR), putative tagatose-6- phosphate kinase (TA6K) from Listeria innocua (PDBid 3Q1Y), and Pfk-2 from E. coli (PDBid 3UMP). A schematic representation of the secondary structural elements formed by this region in Pfk-2 is shown above the alignment. The loops contributing to the metal binding site in the family are depicted in black. Asterisks indicate the positions of the residues that coordinate the monovalent cation in Pfk-2.
