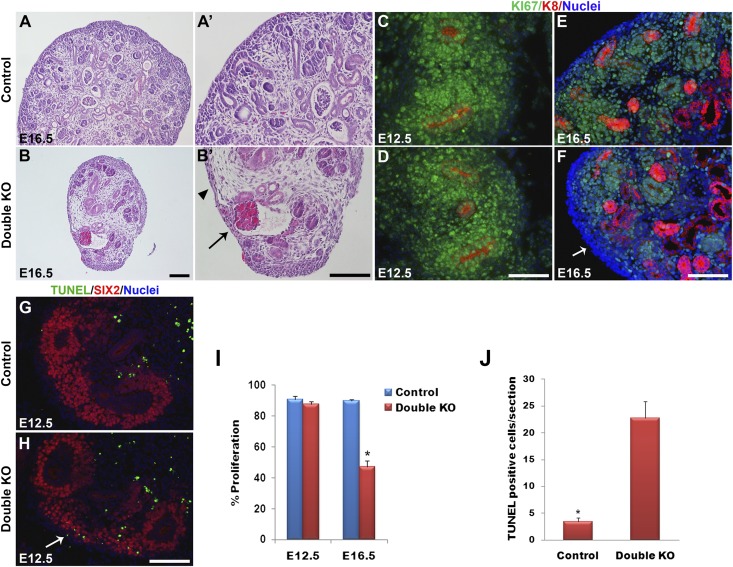
Figure 2.
Defects in renal architecture, cell proliferation, and apoptosis in DKO kidneys. (A and B) Hematoxylin-eosin staining of kidney sections at E16.5. Control kidneys at low (A) and high (A’) magnifications show the MM to be aggregated around the UB tips in the nephrogenic zone in the outer cortex. (B and B’) DKO (Dlg1fl/fl;Caskfl/fl;Pax3-Cre) kidneys showed a striking depletion of MM. Arrowhead points to a zone lacking MM and UB tips. Arrow points to a glomerulus. (C–F) Immunostaining of E12.5 and E16.5 kidneys for Ki67 (green; proliferating cells) and K8 (red; UB derivatives); nuclei are blue. Ki67 staining was similar in E12.5 control (C) and DKO (Dlg1fl/fl;Caskfl/fl;Six2-EGFP/Cre) (D) kidneys but was undetectable in some cortical zones in the E16.5 DKO (Dlg1fl/fl;Caskfl/fl;Pax-3Cre) kidneys (arrow in F) compared with control (E). (G and H) TUNEL staining at E12.5 revealed increased apoptosis in the nephrogenic zone of DKO (Dlg1fl/fl;Caskfl/fl;Six2-EGFP/Cre) kidneys (H) versus controls (G). (I) The percentage of Ki67-positive cells in DKO versus control kidneys was not different at E12.5 but was significantly reduced in DKO kidneys at E16.5 (*P<0.05). (J) TUNEL-positive cells were significantly increased in the DKO kidneys at E12.5 (*P<0.05). Data represent mean values; bars indicate SEM. Scale bars, 100 µm.