Abstract
BACKGROUND
Laser therapy is a low cost, non-invasive procedure with good healing results. Doubts exist as to whether laser therapy action on microorganisms can justify research aimed at investigating its possible effects on bacteria-infected wounds.
OBJECTIVE
To assess the effect of low intensity laser on the rate of bacterial contamination in infected wounds in the skin of rats.
METHODS
An experimental study using 56 male Wistar rats. The animals were randomly divided into eight groups of seven each. Those in the "infected" groups were infected by Staphylococcus aureus MRSA in the dorsal region. Red laser diode (AlGaInP) 658nm, 5J/cm2 was used to treat the animals in the "treated" groups in scan for 3 consecutive days. Samples were drawn before inoculating bacteria and following laser treatment. For statistical analysis we used the nonparametric Wilcoxon (paired data) method with a significance level of p <0.05.
RESULTS
The statistical analysis of median values showed that the groups submitted to laser treatment had low bacterial proliferation.
CONCLUSION
The laser (AlGaInP), with a dose of 5J/cm2 in both intact skin and in wounds of rats infected with Staphylococcus aureus MRSA, is shown to reduce bacterial proliferation.
Keywords: Bacterial infections; Laser therapy, low-level; Rats; Surgical wound infection
Abstract
FUNDAMENTOS
Fundamentos: A terapia a laser é um procedimento de baixo custo, não invasiva e com bom desempenho na cicatrização. As dúvidas existentes quanto a sua ação sobre microrganismos justifica a realização de pesquisas visando investigar os possíveis efeitos em feridas infectadas por bactérias.
OBJETIVO
Avaliar o efeito do laser de baixa intensidade sobre a taxa de contaminação bacteriana em feridas infectadas na pele de ratos.
MÉTODOS
Estudo experimental, utilizando 56 ratos machos Wistar. Os animais foram distribuídos aleatoriamente em oito grupos de sete animais. Nos animais dos grupos lesionados foi realizada uma incisão na região dorsal.Os animais dos grupos infectados foram infectados por Staphylococcus aureus MRSA. os animais dos grupos tratados foram tratados com laser de Diodo vermelho (AlGaInP) 658nm, 5J/cm2 em varredura, durante 3 dias consecutivos. Foi colhida uma amostra antes de inocular as bactérias e outra após o tratamento com laser. Para a análise estatística foram utilizados os testes não paramétricos de Wilcoxon (dados pareados). Considerando como significante p<0,05.
RESULTADOS
Através da análise estatística das medianas, observou-se que os grupos submetidos ao laser apresentavam uma proliferação bacteriana menor.
CONCLUSÃO
O laser (AlGaInP), com uma dose de 5J/cm2, tanto em feridas quanto em pele íntegra de ratos infectados por Staphilococcus aureus MRSA, se mostrou capaz de reduzir a proliferação bacteriana.
INTRODUCTION
Wound healing is the result of a perfect and coordinated cascade of cellular and molecular events that interact to repave and rebuild tissue. Although significant progress has been made in understanding the processes and phenomena involved at different stages of tissue repair, and substantial investment has been made in researching and developing resources and technologies to facilitate these processes, the incidence and prevalence of chronic ulcers remain extremely high.1,2, 3 Bacterial infection, hypoxia and trauma have been shown to be common chronic wound problems.4 Infections caused by Pseudomonas aeruginosa and Staphylococcus aureus are the two most frequently encountered bacteria in pressure ulcers.5 The growth of antibiotic-resistant bacteria has encouraged the development of new strategies to combat infection.6 Healing by using low intensity laser began to be studied in Europe in the 1970s, where positive results were obtained with laser irradiation on cultured cells and in animals.7 It was suggested that the low-energy laser (EBL) increases cell metabolism, stimulates oxidative phosphorylation and reduces local inflammatory response, positively changing electrophysiological properties of the irradiated tissue.8 Low intensity laser application also has the effect of biomodulation, which activates or inhibits the physiological processes and has biochemical and metabolic effects via photochemical and/or photophysical processes. This function varies according to the irradiation parameters (wavelength, intensity and energy dose) employed during the treatment. Lower doses cause stimulation of cellular activity and high doses inhibit cellular growth.9 ,10 A number of studies have been conducted on the effect of laser on infected wounds. Santos et al. for example observed, using histological analysis, the healing of wounds infected with S. aureus when treated with laser at a dose of 5J/cm2.11 The result showed that the animals in the treated group had a higher number of blood vessels, better resolution of inflammation, and collagen fibers that were well-organized compared to the control animals. Guffey et al. concluded, after in vitro study, that the combination of light at a wavelength of 405 m hh me 880 kills Staphylococcus aureus and Pseudomonas sp, suggesting that similar effects occur in bacterial infection in vivo.12 Coutinho, on the basis of in vitro bacteriological study, evaluated the effect of two types of low-level laser energy on different bacterial populations usually present in post-traumatic wounds.13 Under the conditions studied, the application of laser did not increase the number of bacterial colonies.13 Despite advances in the use of low intensity laser on wound healing, the exact biophysical mechanisms of irradiation of LBE in bacterial cells in vivo have not been thoroughly investigated, thus the use of the method remains controversial in cases of suspected or confirmed contamination of soft tissue wounds.14 Our aim was to evaluate the effectiveness of low intensity laser on the healing of infected wounds in rats.
METHOD
The project was submitted and approved by the Ethics Committee of the Federal University of the Vale do São Francisco - 079240509 UNIVASF Protocol and the Federal University of São Paulo - UNIFESP
Sample
We used 56 male Wistar rats weighing 250 to 300g, from the Vivarium of UNIVASF.
Experimental design
The animals were randomly divided into eight groups of seven animals:
Spine Group 1
Spine Group 2 infected
Group 3 not affected and infected
Group 4 injured, infected and treated with laser
Group 5 not affected, infected and treated with laser
Group 6 injured and treated with laser
Group 7 injured and not treated with laser
Group 8 not harmed, not infected and not treated with laser
Weighing, anesthesia and shaving
The rats were weighed and anesthetized with an intraperitoneal injection of ketamine (50mg/ml) solution with 2:1 and xylazine (20mg/ml) respectively at a dose from 0.10 to 0.15 ml/100g body mass. After loss of foot reflex the animals were considered to be anesthetized.
Production of injury
Animals in groups 1, 2, 4, and 6 were subjected to a dorsal incision in the neck with the aid of a metal "punch" 1.0 cm in diameter (Figures 1 and 2). The animals were then randomized into the above-mentioned groups, housed in individual cages and correctly identified.
FIGURE 1.
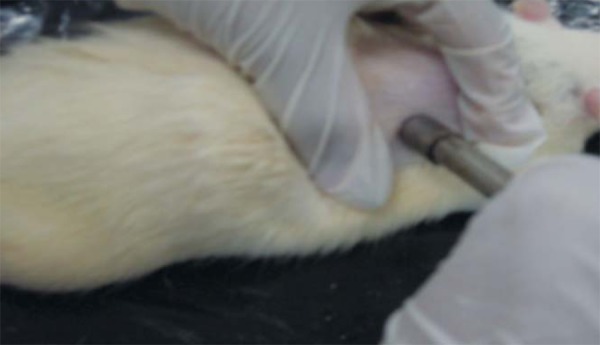
Using the punch
FIGURE 2.
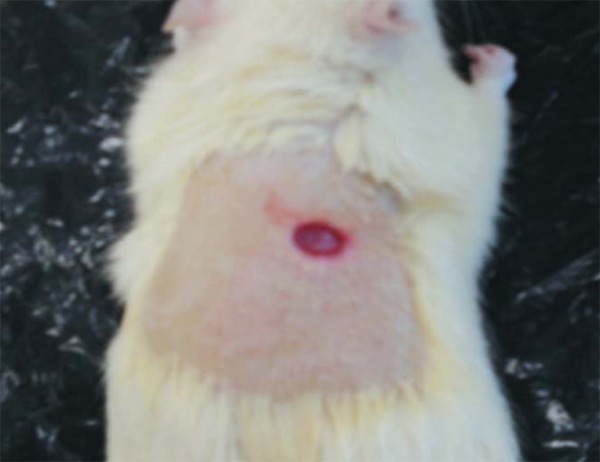
Lesion on the animal's back
Innoculation of microorganisms
The animals in groups 2, 3, 4 and 5 were infected by Staphylococcus aureus grown in the Microbiology and Immunology Animal Laboratory UNIVASF. Bacteria were grown on nutrient agar for an incubation period of 24 hours at 37º C in aerobic conditions. After verifying their viability and purity, the colonies were used for turbidity in BHI broth and incubated for 8 hours. Turbidity was then adjusted in the 0.5 Mac Farland range (1x108UFC/mL). 100 microliters (1x106UFC/mL) of turbidity were applied on the wound and spread with the aid of a sterile swab (Figure 3).
FIGURE 3.
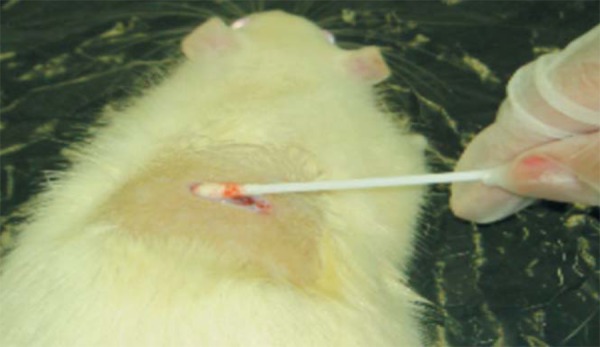
Application of S. aureus
Laser
We used the HTM compact laser device (ANVISA 80212480005), red laser diode AlGaInP), wavelength(l) of 658hm. Laser application began 15 minutes after inoculation of the bacteria and continued for three consecutive days at a dose of 5J/cm2 for 6 minutes and 55 seconds in scan.
Colony count of bacteria
A swab was collected in the UNIVASF microbiology laboratory before starting treatment on day 1 (D1) and another after three laser applications - on day 3 (D3) - to determine the distribution of bacteria. The swab was transported in a sealed test tube containing Stuart transport medium. Counting the number of colonies was undertaken by the Pour Plate method, with incubation periods of 30, 60 and 120 minutes.
Euthanasia
The animals were euthanized by deep anesthesia.
Statistical Analysis
Statistical analysis was performed by Friedman's nonparametric test (paired data) and the nonparametric Wilcoxon test (paired data). All comparisons were considered significant at p <0.05.
RESULTS
Number of colony-forming units in D1 and D3
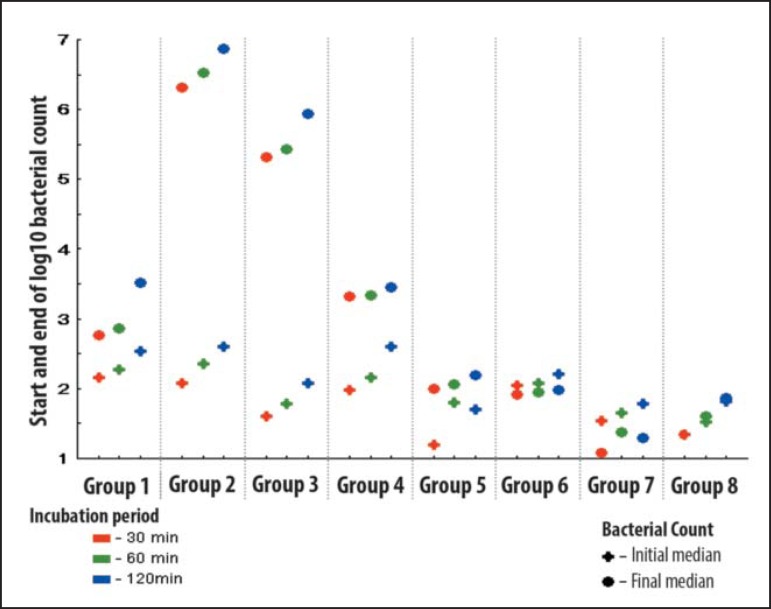
The variable was defined as the logarithm (base 10) of the bacterial count. The comparison between the number of colony-forming units in the three incubation times (30-60-120 minutes) between the D1 and D2 was performed by Friedman's nonparametric test (paired data) and the difference was considered significant. The comparison between the initial and final results in the three incubation times (30-60-120 minutes) was performed by nonparametric Wilcoxon test (paired data) (Graph 1).
GRAPH 1.
Medians of the numbers of colony-forming units at D1 (initial median) and D3 (final median)
Percentage change in bacterial growth.
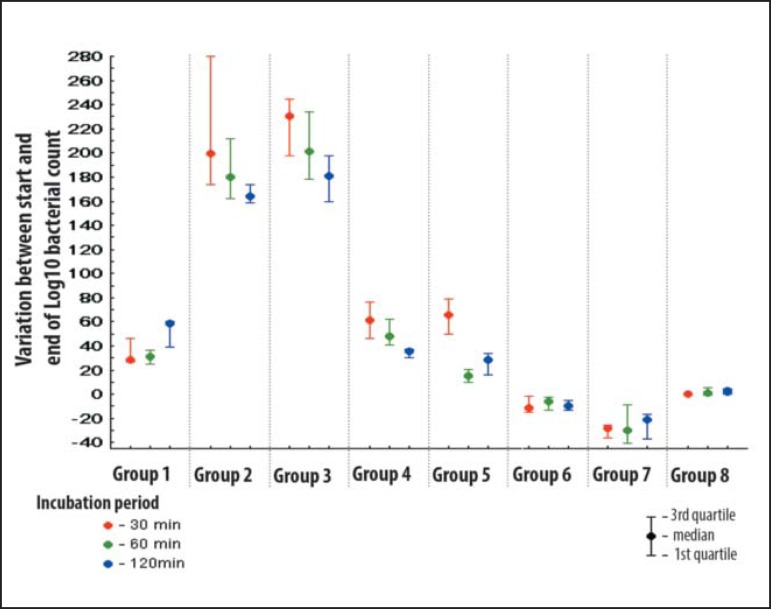
To check the variation in bacterial growth between the D1 and D3 in percentage terms the bacterial variation was defined as follows: Range = log (final bacterial count) - log (initial bacteria count) x 100 log (initial bacteria count) graph 2 shows the percentage change in bacterial growth for each group at the times evaluated (D1 to D3).
GRAPH 2.
Percentage difference in bacterial growth (D1-D3)
Comparison between groups (Wilcoxon test).
We verified that the variation was equal between bacterial groups for each incubation time. In all comparisons significance at <0.05 (Table 1) was considered.
Table 1.
Analysis of variance between bacterial wounds and intact skin at different periods
| Variable Statistics | Incubation time | ||||
| 30 min | 60 min | 120 min | |||
| Group 1 x Group 8 | |||||
| P value | 0.001 | 0.001 | 0.001 | ||
| 5% | rejects H0 | rejects H0 | rejects H0 | ||
| G8 % - G1 % | |||||
| Average | -27.5% | -20.0% | -37.2% | ||
| Median | -28.0% | -23.9% | -37.1% | ||
| Group 2 x Group 3 | |||||
| P value | 0.535 | 0.318 | 0.456 | ||
| 5% | accepted H0 | accepted H0 | accepted H0 | ||
| G3% e - G2 % | |||||
| Average | 30.2% | 21.9% | 17.6% | ||
| Median | 30.5% | 21.2% | 17.0% | ||
| Group 4 x Group 5 | |||||
| P value | 0.535 | 0.001 | 0.097 | ||
| 5% | accepted H0 | rejects H0 | acceptedH0 | ||
| G5% - G4% | |||||
| Average | 1.1% | -32.8% | -7.1% | ||
| Median | 4.2% | -33.3% | -7.3% | ||
| Group 6 x Group 7 | |||||
| P value | 0.001 | 0.097 | 0.001 | ||
| 5% | rejects H0 | rejects H0 | rejects H0 | ||
| G7% - G6% | |||||
| Average | -21.3 | -14.8 | -17.6 | ||
| Median | -17.2 | -24.0 | -11.6 | ||
| Group 6 x Group 8 | |||||
| P value | 0.026 | 0.001 | 0.001 | ||
| 5% | rejects H0 | rejectsH0 | rejects H0 | ||
| G8% - G6% | |||||
| Average | 8.9% | 13.8% | 10.7% | ||
| Median | 11.3% | 7.7% | 11.6% | ||
DISCUSSION
Through statistical analysis of the medians, it was observed that in groups 1, 2, 3, 4 and 5 statistically significant bacterial growth was present. However the groups submitted to laser treatment presented a lower growth. In vivo and in vitro tests showed that the energy of the laser wavelengths and different regimes of irradiation have a bactericidal effect on Staphylococcusaureus 4.15,16,17,18,19 Published studies show that the inhibitory effect of the laser depends, among other factors, on the wavelength, power and the dose employed. Using higher doses would have a greater destructive effect.9,19,20 Nussbaum et al. reaffirmed this finding by investigating the in vitro bacterial growth of Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli when irradiated with laser of 630 m h, h 660 m, 810 I 905 hh m (doses ranging from 1J/cm2 and 50J / cm2). The results showed the existence of different values of wavelength and doses for inhibiting each type of bacteria 4. The S. aureus dose that resulted in greater inhibition of bacterial growth was 5J/cm2 4 for the wavelengths studied. The 5J/cm2 dose also proved effective for healing wounds infected with S. aureus, according to Santo et al.11 This research conformed to the literature since, when laser h 658 m was used at a dose of 5J/cm2, the treated groups were observed to have lower bacterial growth. Pressure ulcers affect many patients and their incidence and prevalence is higher in the ICU. The contraction of a wound can be influenced by certain factors, including the main infection.3,4,21 The results of this study show a greater increase in bacterial growth in wounds and less growth in intact skin, as suggested by the literature (Table 1). This result was statistically significant (p = 0.001), for wounds favoring bacterial growth. As for the reduction of bacterial growth using the laser, Coutinho et al. suggest that in addition to the dose, potency and wavelength used for the bacteria under study, the culture medium of this bacterium can be crucial to its growth (i.e. the in vitro conditions may not match the in vivo conditions).13 Analyzing the bacterial growth in wounds, Jawhara and Mordon in 2006 applied laser h 810 m in wounds infected with Escherichia coli, observing a gradual decrease in the number of bacteria and their disappearance in 48 hours. According to this research, the laser dried the wound, rendering it inhospitable to bacterial growth.6 As for infected wounds in rats evaluated by histological and immune histochemical methods, Ferreira et al. found a larger number of lymphocytes and macrophages in lesions treated with laser 632.8 hm. This demonstrated that laser therapy increases the phagocytic capacity of macrophages, facilitating cleaning of the wound.22 Although the literature questions the influence of the environment around the bacteria and the application of laser in such circumstances, our study did not observe statistically significant differences when comparing the use of lasers in intact skin and wounds, resulting in a significant reduction in both cases.6,13,21,22
CONCLUSION
The red laser diode (AsGaAlP), wavelength 658 h m, when employing a dose of 5J/cm2 in wounds and intact skin of rats infected with Staphylococcus aureus MRSA, has been shown to reduce bacterial growth. The application of laser in wounded and intact skin may be useful in the treatment and / or prevention of infection in high risk patients.
Footnotes
Financial support: none.
Conflict of interests: none.
* Study undertaken in the Microbiology Laboratory, Agricultural Sciences Foundation Campus of the Federal University of the Vale do São Francisco Foundation (UNIVASF) - Juazeiro (BA), Brazil.
REFERENCES
- 1.Mandelbaum SH, Di Santis EP, Mandelbaum MHS. Cicatrização: conceitos atuais e recursos auxiliares - Parte I. An BrasDermatol. 2003;78:393–410. [Google Scholar]
- 2.Mandelbaum SH, Di Santis EP, Mandelbaum MHS. Cicatrização: conceitos atuais e recursos auxiliares - Parte II. An Bras Dermatol. 2003;78:525–42. [Google Scholar]
- 3.Louro M, Ferreira M, Póvoa P. Avaliação de protocolo de prevenção e tratamento de úlceras de pressão. Rev Bras TerIntensiva. 2007;19:337–41. [PubMed] [Google Scholar]
- 4.Nussbaum EL, Lilge L, Mazzulli T. Effects of 630 - 660- 810- and 905-ηµ laser irradiation delivering radiant exposure of 1-50 j/cm2 on three species of bacteria in vitro. J Clin Laser Med Surg. 2002;20:325–33. doi: 10.1089/104454702320901116. [DOI] [PubMed] [Google Scholar]
- 5.Benvindo RG, Braun G, Carvalho AR, Bertolini GRF. Efeitos da terapia fotodinâmica e de uma única aplicação de laser de baixa potência em bactérias in vitro. Fisioter Pesq. 2008;15:53–7. [Google Scholar]
- 6.Jawhara S, Mordon S. Monitoring of bactericidal action of laser by in vivo imaging of bioluminescent E. coli in a cutaneous wound infection. Lasers Med Sci. 2006;21:153–9. doi: 10.1007/s10103-006-0388-8. [DOI] [PubMed] [Google Scholar]
- 7.Rocha JAM, Oliveira RG, Farias RE, Andrade LCF, Aarestrup FM. Modulação da proliferação fibroblástica e da resposta inflamatória pela terapia a laser de baixa intensidade no processo de reparo tecidual. An Bras Dermatol. 2006;81:150–156. [Google Scholar]
- 8.Rocha JAM, Vieira BJ, Andrade LCF, Aarestrup FM. Effects of low-level laser therapy on the progress of wound healing in humans: the contribution of in vitro and in vivo experimental studies. J Vasc Bras. 2007;6:257–66. [Google Scholar]
- 9.Henriques ACG, Cazal C, JFL Ação da laserterapia no processo de proliferação e diferenciação celular: revisão da literatura. Rev Col Bras Cir. 2010;37:295–302. doi: 10.1590/s0100-69912010000400011. [DOI] [PubMed] [Google Scholar]
- 10.Damante CA, Marques MM, Micheli G. Terapia com laser em baixa intensidade na cicatrização de feridas: revisão de literatura. RFO. 2008;13:88–9. [Google Scholar]
- 11.Oliveira RF, Oliveira DAAP, Monteiro W, Zangaro A, Magini M, Soares CP. Comparison Between the effect of low-level laser therapy and low-intensity pulsed Ultrasonic irradiation in vitro. Photomed Laser Surg. 2008;26:6–9. doi: 10.1089/pho.2007.2112. [DOI] [PubMed] [Google Scholar]
- 12.Meral G, Tasar F, Kocagöz S, Sener C. Factors affecting the antibacterial effects of Nd:YAG laser in vivo. Lasers Surg Med. 2003;32:197–202. doi: 10.1002/lsm.10128. [DOI] [PubMed] [Google Scholar]
- 13.Coutinho F, Giordano V, Santos CM, Carneiro AF, Amaral N P, Touma MC, et al. O efeito do laser de baixa energia no crescimento bacteriano "in vitro". Rev Bras Ortop. 2007;42:248–53. [Google Scholar]
- 14.Busnardo V L, Biondo-Simões MLP. Os efeitos do laser hélio-neônio de baixa intensidade na cicatrização de lesões cutâneas induzidas em ratos. Rev Bras Fisioter. 2010;14:45–51. [PubMed] [Google Scholar]
- 15.Fukuda TY, Malfatti CA. Análise da dose do laser de baixa potência em equipamentos nacionais. Rev Bras Fisioter. 2008;12:70–4. [Google Scholar]
- 16.Ali E, Mohammad B, Sodabe T, Zhaleh M, Shahid B, Tehran I. Low-level laser therapy with pulsed infrared laser accelerates third-degree burn healing process in rats. J Rehabil Res Dev. 2009;46:543–54. doi: 10.1682/jrrd.2008.09.0121. [DOI] [PubMed] [Google Scholar]
- 17.Kaya GŞ, Kaya M, Gürsan N, Kireççi E, Güngörmüş M, Balta H. The use of 808-ηµ light therapy to treat experimental chronic osteomyelitis induced in rats by methicillin- resistant Staphylococcus aureus. Photomed Laser Surg. 2011;29:405–12. doi: 10.1089/pho.2010.2807. [DOI] [PubMed] [Google Scholar]
- 18.Krespi YP, Kizhner V, Nistico L, Hall-Stoodley L, Stoodley P. Laser disruption and killing of methicillin-resistant Staphylococcus aureus biofilms. Am J Otolaryngol. 2011;32:198–202. doi: 10.1016/j.amjoto.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Dadras S, Mohajerani E, Eftekhar F, Hosseini M. Different Photoresponses of Staphylococcus aureus and Pseudomonas aeruginosa to 514, 532, and 633 nmlow level lasers in vitro. Current microbiology. 2006;53:282–6. doi: 10.1007/s00284-005-0490-3. [DOI] [PubMed] [Google Scholar]
- 20.Peplow PV, Chung TY, Baxter GD. Laser photobiomodulation of wound healing: a review of experimental studies in mouse and rat animal models. Photomed Laser Surg. 2010;28:291–325. doi: 10.1089/pho.2008.2446. [DOI] [PubMed] [Google Scholar]
- 21.Reddy M, Gill SS, Kalkar SR, Wu W, Anderson PJ, Rochon PA. Treatment of pressure ulcers: a systematic review. JAMA. 2008;300:2647–62. doi: 10.1001/jama.2008.778. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira MC, Gameiro J, Nagib PR, Brito VN, Vasconcellos Eda C, Verinaud L. Effect of low intensity helium-neon (He-Ne) laser irradiation on experimental paracoccidioidomycotic wound healing dynamics. Photochemistry and Photobiology. 2009;85:227–33. doi: 10.1111/j.1751-1097.2008.00423.x. [DOI] [PubMed] [Google Scholar]