Abstract
Testes are sensitive to toxicants, such as cadmium and phthalates, which disrupt a local functional axis in the seminiferous epithelium known as the “apical ectoplasmic specialization (apical ES)-blood-testis-barrier (BTB)-basement membrane (BM)”. Following exposure, toxicants contact the basement membrane and activate the Sertoli cell, which perturbs its signaling function. Thus, toxicants can modulate signaling and/or cellular events at the apical ES-BTB-BM axis, perturbing spermatogenesis without entering the epithelium. Toxicants also enter the epithelium via drug transporters to potentiate their damaging effects, and downregulation of efflux transporters by toxicants impedes BTB function such that toxicants remain in the epithelium and efficiently disrupt spermatogenesis. These findings support a novel model of toxicant-induced disruption of spermatogenesis that could be interfered with using small molecules.
Toxicant exposure and decreasing male fertility
Recent studies have linked the declining reproductive health and fertility in men with toxicants found in the environment, in particular endocrine disrupting chemicals (EDCs) such as cadmium, bisphenol A, and phthalates. These compounds are widespread in our environment and are integrated components of our food chain through industrial activities in nations across the globe [1–8]. Beyond mere exposure, declining fertility is likely due to the sensitivity of the mammalian testis to these toxicants [2, 7, 9], which was first reported ~60 years ago for male rodents when it was observed that cadmium induced testicular injury and male sterility [10, 11]. Nearly 50 years ago, the blood–testis barrier (BTB) was found to be more sensitive to cadmium toxicity than the endothelial tight junction (TJ) barrier of the blood vessels [12]. For instance, when the kinetics of BTB and blood vessel disruption were compared in the same group of rats following treatment with a single dose of cadmium chloride, BTB disruption was detected at least 14-hr before any signs of blood vessel damage were detected [13, 14]. However, the molecular mechanism(s) underlying toxicant-induced male infertility and the reasons for the vulnerability of the testis to toxicants remain largely unknown.
Recent studies have begun to characterize the “apical ectoplasmic specialization (apical ES) – blood-testis barrier (BTB) – basement membrane (BM)” functional axis in the seminiferous epithelium, an axis which regulates and coordinates cellular events that occur across the epithelium during the seminiferous epithelial cycle of spermatogenesis [15–19] (see Figure 1). For instance, it has been shown during spermiation that biologically active fragments derived from the laminin chains released at the apical ES (see Glossary) perturb the BTB (see Box 1) and hemidesmosome function [15, 18]. Furthermore, a loss of hemidesmosome function through the downregulation of β1-integrin expression ( β1-integrin and α2-laminin form a functional adhesion complex at the hemidesmosome) has also been shown to disrupt the BTB [15]. Collectively, these changes coordinate the events of spermiation for sperm release [20] and BTB restructuring to accommodate the transit of preleptotene spermatocytes across the barrier [21], events that occur simultaneously at stage VIII of the epithelial cycle (Figure 1). Studies of mice treated with mono-(2-ethylhexyl) phthalate (MEHP) experience testicular injury mediated by disruption of the apical ES-BTB-BM axis [22–24] and illustrate that this axis is a target of environmental toxicants [25]. In this article, we critically evaluate the likely mechanism(s) by which toxicants impede testicular function, most notably via the BTB. We note that some environmental toxicants, such as cadmium, can exert their disruptive effects by perturbing cell adhesion proteins at the base of the Sertoli cells, including as the Ca2+-dependent cell adhesion proteins cadherins, by competitive binding to Ca2+-binding domain [26–28]. It is also possible that some EDCs, such as phthalates and bisphenol A, exert their effects by disrupting the Leydig cell steroidogenesis in the interstitium [29–32] by perturbing the estrogen- and androgen-mediated functions in the testes necessary to maintain spermatogenesis, including BTB function [33, 34]. However, the findings and concept that environmental toxicants disrupt endocrine regulation in mammalian body, which leads to multiple pathological conditions including male reproductive dysfunction, have recently been reviewed in several excellent articles [35–41] and are not discussed here.
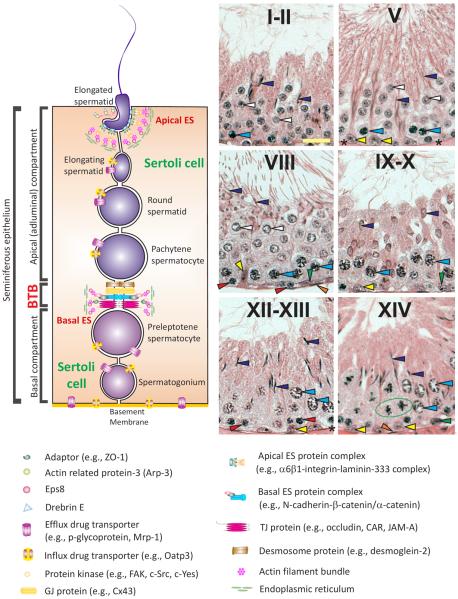
Figure 1. Relative location of apical and basal ES in the seminiferous epithelium of mammalian testes and their physiological relationship with spermatids and Sertoli cells during the epithelial cycle of spermatogenesis.
BTB, located near the basement membrane, physically divides the seminiferous epithelium into the basal and the apical (adluminal) compartments. Co-existing tight junctions (TJs), gap junctions (GJs), and basal ES together with desmosomes constitute the BTB in the testis. The most notable and unique feature of the BTB is the presence of tightly packed actin filament bundles that lie perpendicular to the Sertoli cell plasma membrane and sandwiched between the endoplasmic reticulum (ER) and the apposing Sertoli cell plasma membranes. The apical ES, a testis-specific adherens junction (AJ), also shares similar ultrastructural features as of the basal ES at the BTB except that that tightly packed actin filament bundles were only found in the Sertoli cell and no visible ultrastructures were seen in the elongating/elongated spermatid. Also, apical ES do not co-exist with any other junctions at the spermatid-Sertoli cell interface. Once it appears in step 8 spermatids, it is the only anchorage device, replacing desmosome and GJ, to confer adhesion and polarity to developing spermatids until step 19 spermatids when it undergoes degeneration at spermiation. Influx and efflux drug transporters are also found in Sertoli cells, spermatogonia, spermatocytes and spermatids that regulate the movement of xenobiotics ”in” and “out” of the seminiferous epithelium. In rodent and human testes, spermatogenesis can be defined into different stages, such as I–XIV, I–XII and I–VI in the rat, mouse, and human, respectively. The micrographs shown in B depict the selected stages of the epithelial cycle of spermatogenesis in the rat testis of I–II, V, VIII, IX–X, XII–XIII and XIV to better illustrate the concept of the epithelial cycle of spermatogenesis. It is noted that meiosis takes place in the seminiferous epithelium [note: the epithelium is constituted by Sertoli cells (see “yellow” arrowheads in any micrograph in this panel) and germ cells at different stages of their development, see colored arrowheads in these micrographs, which lie on the basement membrane (see “asterisks” in V and XII–XIII and the “orange” arrowheads annotating the peritubular myoid cells lying behind the basement membrane)] at stage XIV of the epithelial cycle (see the “green circle” enclosing the meiotic germ cell undergoing anaphase), which gives rise to round spermatids. Spermatids (step 1 and 2 in stage I and II of the epithelial cycle, see “white” arrowheads in I–II) develop progressively via spermiogenesis through I–XIV until they become step 14 spermatids at stage XIV, which further develop via I–VIII (see “purple” arrowheads in I–II which are step 15–16 spermatids) of the cycle until they eventually transform to step 19 spermatids at stage VIII (see “purple” arrowheads in VIII, which are step 19 spermatids that line up at the luminal edge of the seminiferous tubule lumen) when the release of fully developed spermatids (i.e., spermatozoa) occur at stage VIII of the epithelial cycle via spermiogenesis. At stage VIII of the cycle when spermiation takes place, BTB restructuring occurs so that preleptotene spermatocytes (see “red” arrowheads in VIII) also traverse the BTB to enter the adluminal compartment to prepare for meiosis I/II that occurs at XIV of the epithelial cycle. In short, the apical ES-BTB-basement membrane axis discussed herein coordinate many of these events that occur during the epithelial cycle, however, this axis also becomes a target of environmental toxicants that induce male reproductive dysfunction in men. Scale bar = 25 μm in the micrograph at stage I–II of the cycle, which applies to other micrographs in this panel. Yellow arrowhead, Sertoli cell; orange arrowhead, peritubular myoid cell; blue arrowhead, pachytene spermatocyte; asterisk, basement membrane. In a I–II tubule, white arrowhead, step 1–2 spermatid; purple arrowhead, 15–16 spermatid. In a V tubule, white arrowhead, step 5 spermatid; purple arrowhead, step 17 spermatid. In a VIII tubule, white arrowhead, step 8 spermatid; purple arrowhead, step 19 spermatid; red arrowhead, preleptotene spermatocyte; green arrowhead, type A spermatogonium. In a IX–X tubule, purple arrowhead, step 9–10 spermatid; green arrowhead, type A spermatogonium. In a XII–XIII tubule, purple arrowhead, step 12–13 spermatid; red arrowhead, zygotene spermatocyte. In a XIV tubule, purple arrowhead, step 14 spermatid; green arrowhead, type A spermatogonium.
Ectoplasmic specialization
When cross-sections of testes are examined using electron microscopy, the most notable ultrastructural feature at the Sertoli–Sertoli and the Sertoli–germ cell interface, across the entire seminiferous epithelium, are the bundles of actin filaments that lie perpendicular to the apposing Sertoli cell plasma membranes at the BTB and the Sertoli-elongating/elongated spermatid plasma membranes. These membranes are designated the apical and the basal ectoplasmic specializations (ES; see Glossary), respectively (Figure 1). Furthermore, these actin filament bundles are sandwiched between cisternae of endoplasmic specialization and the apposing plasma membranes (Figure 1). These features are found on both sides of the Sertoli cells in the basal ES, but limited only to the Sertoli cell at the apical ES (Figure 1). The basal ES coexists with either a tight junction (TJ) or gap junction (GJ), which together with the desmosome constitute the BTB. These actin filament bundles strengthen the BTB, making it one of the tightest blood-tissue barriers in the mammalian body [21, 42–44]. However, once the apical ES appears at the Sertoli cell-spermatid interface (step 8–19 in the rat testis) (see Figure 1), it is the only anchorage device, replacing desmosomes and GJs found between Sertoli cells and step 1–7 spermatids [21, 44, 45]. Interestingly, whereas the apical ES is considered to be an actin-based AJ, its constituent adhesion molecules are composed of proteins usually restricted to other adhesion complexes, such as non-actin based AJs (e.g., N-cadherin, nectins), TJs (e.g., CAR, JAM-A), GJs (e.g., connexin-43), desmosomes (e.g., desmoglein-2), and focal adhesion complexes (e.g., α6β1-integrin, laminin-α3β3γ3 chains). Thus, the ES is a hybrid junction [46, 47]. Its adhesion force is stronger than desmosomes [48], which are found extensively in the skin and were previously considered to be the strongest anchoring junction in mammalian tissues [49, 50].
Surprisingly, the actin filament bundles at the basal ES are exceedingly vulnerable to toxicants. Cadmium chloride [13, 51] or glycerol [52] treatments in rats induces truncation and defragmentation of actin filaments at the basal ES, which in turn contributes to irreversible disruption of the BTB and leads to infertility. Furthermore, during the seminiferous epithelial cycle of spermatogenesis, these actin filament bundles at the apical and basal ES structures undergo cyclic re-organization from “bundled” to “de-bundled” configurations to restructure the ES junctions and facilitate spermatid movement across the epithelium at spermiogenesis and the transit of preleptotene spermatocytes at the BTB, respectively. This novel mechanism, crucial to streamline cellular events in the testes during the epithelial cycle, is targeted by the toxicants to perturb spermatogenesis, thereby making the testis one of the most vulnerable organs to toxicant-mediated injury.
The apical ES-BTB functional axis: a molecular target of toxicants in the testis
The apical ES-BTB axis coordinates and regulates cellular events across the seminiferous epithelium [15], a model supported by studies using a Sertoli cell injury model in which rodents were treated with MEHP [22, 23]. It has been suggested that at stage VIII of the epithelial cycle, the apical ES undergoes degeneration to allow the release of mature sperm at spermiation [20]. This release is mediated via the action of matrix metalloprotease-2 (MMP-2), which cleaves cell adhesion protein complexes at the site of release, such as α6β1-integrinlaminin-333 [15, 22, 23, 53]. MMP2 cleavage releases biologically active laminin fragments, which serve as autocrine factors that disrupt the BTB via changes in vesicle-mediated protein endocytosis [15] to facilitate the transit of preleptotene spermatocytes at the immunological barrier [15, 23, 54]. Similar molecular events occur in other organs, where fragments of laminin chains are biologically active peptides that can modulate barrier function, such as the TJ barrier of microvessels (for a review, see [55]). The net result of these changes coordinate the events of spermiation and BTB restructuring, which are the two distinctive cellular events that occur at the opposite ends of the seminiferous epithelium at stage VIII of the epithelial cycle (Figure 2). In short, at stage VIII of the epithelial cycle the tightly organized actin filaments at the apical and the basal ES, residing at the opposite ends of the epithelium, are disorganized simultaneously to coordinate these two distinct cellular events. However, the underlying mechanism(s) that facilitates this event remains unknown. But recent studies using a toxicant model in which adult rats were treated with adjudin [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] to induce premature release of spermatids and BTB restructuring [56–60] have yielded insights into the mechanisms of this event.
Figure 2. Schematic illustrating the model mechanism by which toxicants induce reproductive dysfunction via changes in the configuration of the actin filament bundles.
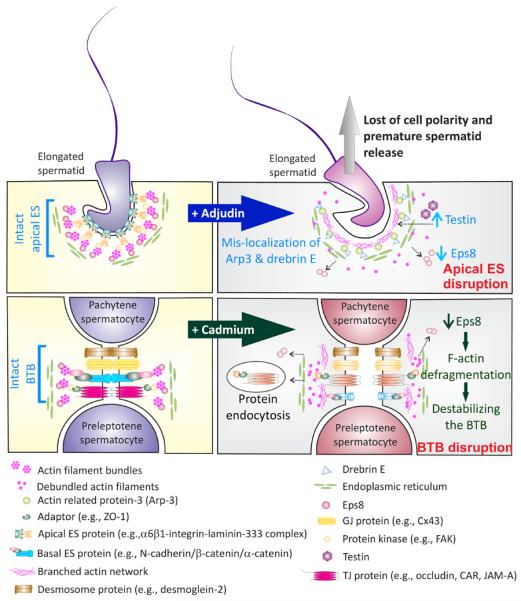
At stage VII of the epithelial cycle (left panel), spermatid and Sertoli cell adhesion is maintained by actin filament bundles at the ES at both the Serotli-spermatid (i.e., apical ES) and Sertoli-Sertoli (i.e., basal ES) cell interface. Exposure of the testis to toxicants, such as adjudin, downregulates Eps8, a barbed end capping and bundling protein of actin filaments, which coupled with mis-localization of Arp3 (a component of the Arp2/3 protein complex which together with N-WASP induces branched actin polymerization, creating a branched actin network, destabilizing the ES) and drebrin E (an actin binding protein that recruits Arp3 to a specific cellular domain) (right panel). A surge in the expression of testin, an actin-binding protein, is also noted. The combined effects of these changes cause a conversation of the F-actin the apical ES from a “bundled” and a “debundled” state, destabilizing the apical ES, leading to a loss of spermatid adhesion and polarity, analogous to spermiation. This thus leads to spermatid depletion from the epithelium, impeding sperm counts in men. Similarly, cadmium reduces Eps8 levels at the BTB, inducing “de-bundling” of actin filaments at the basal ES, which in turn destabilizes BTB, together with an increase in TJ- and/or basal ES- protein internalization by endocytosis. Cadmium exposure thus disrupts the BTB and earlier studies have shown that this disruption is irreversible [12, 14, 51]. Recent studies have shown that spermatogenesis fails to reinitialize in rodents when a functional BTB is absent even if there are spermatogonial stem cells/spermatogonia [56, 93].
Adjudin is a potential male contraceptive that induces germ cell loss from the seminiferous epithelium by exerting its effects at the ES, in particular the actin filament bundles [61–63]. Treatment of adult rats with adjudin (50 mg/kg b.w., by gavage) rapidly downregulates the expression of protein Eps8 (epidermal growth factor receptor pathway substrate 8), which is responsible for actin barbed end capping and bundling, at the apical and the basal ES [59, 64], thereby impeding the ability of the ES to maintain the complex network of actin filament bundles at these sites. These changes have also been shown to be coupled with a mis-localization of Arp3 (actin-related protein 3), which together with Arp2 forms the Arp2/3 complex that induces branched actin polymerization or barbed end branching, shifting the “bundled” actin filament to a “branched” and “de-bundled” configuration. This shift destabilizes both the apical and basal ES structures [58]. Furthermore, these changes in Arp3 distribution in the epithelium are further assisted by drebrin E, an actin-binding protein with a strong affinity for Arp3, via its mis-localization at the apical ES and an up-regulation at the basal ES following treatment of rats with adjudin [65]. These combined changes, namely a loss of the actin barbed end capping/bundling protein Eps8 plus an increase in barbed end branching activity via an overexpression and mis-localization of Arp3 and drebrin E, induce re-organization of the actin filament bundles at the apical and the basal ES, leading to spermatid loss from the epithelium and BTB disruption [66].
However, disrupting the BTB requires a longer exposure to adjudin because the basal ES comprises two series of actin filament bundles, one on each side of the adjacent Sertoli cells versus the single series of actin filament bundles at the apical ES, making the basal ES stronger than the apical ES junction (Figure 1). Indeed, treating adult rats with adjudin (50 mg/kg b.w., by gavage) is associated with rapid defragmentation of actin filaments at the apical ES and a loss of spermatid polarity, which occur within hours and prior to the loss of germ cells from the seminiferous epithelium [60]. Yet the BTB is not disrupted, even transiently, until after approximately 2–4 wk of treatment and it is “resealed” by 6-wk [56], illustrating that the actin filaments at the BTB are more resistant to the toxic effects of adjudin. Similarly, treating adult rats with cadmium chloride, which induces BTB disruption and germ cell loss, is also associated with actin filament defragmentation [51]; actin filament bundles are no longer found along the TJs at the BTB to support the barrier function [14]. This is coupled with a loss of spermatid polarity as the heads no longer point toward the basement membrane but are aligned randomly in the epithelium prior to spermatid/germ cell loss from the epithelium [7]. Collectively, these findings suggest that disruption of the acting filament bundles at the ES is a mechanistic pathway by which toxicants such as adjudin and cadmium, and perhaps others, induce effective germ cell loss from the epithelium, disrupting the apical ES-BTB functional axis (Figure 2), and thereby perturbing spermatogenesis.
Recent studies have identified the likely signaling molecules that serve as the “molecular switches” that modify the barbed end actin branching activity of the Arp2/3 complex on preexisting actin filaments, causing “de-bundling” of actin filaments to form a branched actin network. In this context, it is important to note that the conversion of actin filaments from the bundled to the branched configuration may not necessarily disrupt the ES. If this reorganization occurs in a highly restricted cellular domain and in coordination with Eps8 activity, it may be sufficient to maintain the dynamic nature of the BTB even in a “non-restructuring state”, unlike the events that occur around the spermatid head at the apical ES at spermiation when Eps8 is down-regulated.
FAK is a molecular switch of the apical ES-BTB-BM functional axis Studies have shown that FAK (focal adhesion kinase), a major integrin signaling mediator at the focal adhesion complex in most epithelia [58, 67], is an integrated component of the ES [68, 69]. The predominant activated (i.e., phosphorylated) forms of FAK at the apical ES and the basal ES are p-FAKTyr397 and p-FAK-Tyr407, respectively, and these two forms of FAK display highly restrictive spatiotemporal expression at the ES during the epithelial cycle [17, 68]. Interestingly, studies using phosphomimetic (i.e., FAKTyr397Glu, FAK Tyr407Glu) and non-phosphorylatable (i.e., FAK Tyr397Phe, FAK Tyr407Phe) FAK mutants have shown that p-FAK-Tyr397 and p-FAKTyr407 have antagonistic effects on the Sertoli cell TJ-permeability barrier function [17]; p-FAKTyr407 promotes basal ES function, tightening the TJ-barrier, whereas p-FAK-Tyr397 promotes apical ES function but has a disruptive effect on the Sertoli cell TJ-permeability barrier function [17]. Most importantly, p-FAK-Tyr407 exerts its effects by promoting actin polymerization, perhaps inducing the formation of actin filament bundles at the basal ES [17]. These findings illustrate that the restrictive spatiotemporal expression of p-FAK-Tyr397 and -Tyr407 at the apical and the basal ES in the seminiferous epithelium during the epithelial cycle serve as the molecular switches to promote the alteration of F-actin organization by the Arp2/3 complex. In this context, it is interesting to note that the signaling molecules regulating Eps8 remain unknown. However, recent studies have reported that the treatment of Sertoli cells in vitro or adult rats in vivo with cadmium chloride led to a downregulation of p-FAK-Tyr397, suggesting that cadmium perturbs testicular function, at least in part, via its effects on p-FAK-Tyr397 at the apical ES-BTB functional axis.
An emerging concept of toxicant-induced testis injury mediated by changes in F-actin organization
Figure 2 depicts a current model based on recent findings in the field regarding the effects of toxicants (e.g., adjudin, cadmium) on F-actin organization and the F-actin regulatory proteins (e.g., Arp3, Eps8, drebrin) [58, 59, 70, 71] which impedes spermatid adhesion at the apical ES and basal ES function at the BTB. Thus, it is of interest to examine the mechanism(s) by which toxicants can gain access beyond the BTB, which is located near the basement membrane (Figure 1) and effectively blocks biomolecules and toxicants from entering the adluminal compartment. More than a dozen drug transporters are found in the testis, including both efflux and influx drug pumps [21, 72–74]. Many of these transporters, such as P-glycoprotein (an efflux transporter), are highly expressed by Sertoli cells as well as spermatogonia, spermatocytes, and spermatids, effectively “guarding” the testis [73, 75]. These drug transporters prevent toxicants from entering or concentrating in the seminiferous epithelium, protecting the overall integrity of the testis and spermatogenesis [21, 76]. Sertoli cells cultured in vitro that have established a TJ-permeability barrier mimicking the in vivo BTB are found to downregulate the expression of efflux drug transporters P-glycoprotein and Mrp1 (multidrug resistance related protein 1) when treated with cadmium [77]. Treatment of the Sertoli cell epithelium in vitro by adjudin also downregulated the expression of Mrp1, but not P-glycoprotein [73]. These findings suggest that when rodents, and perhaps humans, are exposed to environmental toxicants (e.g., cadmium) or adjudin, these toxicants exert their initial effects by downregulating the expression of efflux drug transporters at the Sertoli cell BTB, destroying the most important line of defense in the testis. Toxicants can then exert effects at the F-actin-rich ultrastructures, namely the ES, by downregulating the expression of actin bundling proteins (e.g., Eps8), upregulating the expression of actin binding proteins (e.g., drebrin E), and inducing the mislocalization of branched actin polymerizating proteins (e.g., Arp2/3 complex), causing “de-bundling” of the actin filaments at the ES, which dismantles the apical ES function first, followed by the basal ES. It is noted that much research is needed to understand the underlying mechanism(s) subsequent to toxicant exposure that reduce the expression of actin-regulatory proteins (e.g., Eps8, Arp3) at the transcriptional level. Nonetheless, this model offers new insights into the therapeutic management of toxicant-induced testicular dysfunction. Given that a recent report has identified the likely interacting (or docking) domain in P-glycoprotein for adjudin [78], it may be possible to design small-molecule inhibitors to block the access of cadmium to P-glycoprotein.
The BTB-basement membrane axis – the molecular target of toxicants in the testis
As noted in Figure 1 and Box 1, the BTB created by adjacent Sertoli cells in the testis is located near the basement membrane; the endothelial TJ-barrier in the microvessels at the interstitium contributes virtually no barrier function. The BTB also physically segregates the seminiferous epithelium into the basal and adluminal compartment so that preleptotene spermatocytes residing at the basal compartment during stage VIII of the epithelial cycle can traverse the BTB to enter the adluminal compartment for further development. Due to this intimacy between the BTB and the basement membrane (which is a modified form of extracellular matrix in the testis [79]), crosstalk is likely between the two ultrastructures. Indeed, biologically active laminin peptides released from the apical ES as the result of proteolytic cleavage of the laminin-γ3 or -β3 chains from the α6β1-integrin-laminin-333 adhesion protein complex [15, 53, 80] by MMP-2 at spermiation [23, 53] perturb hemidesmosome function by downregulating β1-integrin in addition to perturbing the BTB function [15, 18]. More importantly, a disruption of the hemidesmosome function is also found to further perturb the Sertoli cell TJ-permeability barrier. In Sertoli cell epithelium without any detectable apical ES, decreasing β1-integrin expression by 80–% using specific siRNA duplexes [81], significantly perturbed the Sertoli TJ barrier function [15], illustratating the presence of a functional axis between the BTB and the basement membrane. Furthermore, collagen chains found in the basement membrane can also be cleaved via the action of MMP-9 released from Sertoli and/or germ cells near the basement membrane, which can also modulate the function of the Sertoli cell TJ-permeability barrier [82] and supporting the concept of a BTB-basement membrane functional axis. Given that cadmium exposure upregulates the expression of cathepsin L (a cysteine protease and a Sertoli cell product) and MMP-2 [14], and MEHP induces expression of MMP-2 [23], it is likely that these toxicants also mediate testicular injury by disrupting hemidesmosome function, in turn disrupting the BTB as depicted in the model shown in Figure 3.
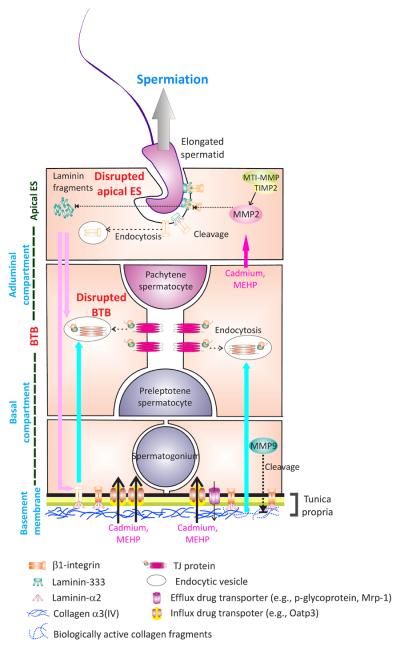
Figure 3. An autocrine-based apical ES-BTB-basement membrane axis regulates spermiation and BTB restructuring in the rat testis.
At spermiation, upon the activation of MMP2 by MTI-MMP and TIMP2 complex, the laminin/integrin adhesion protein complex at the apical ES is cleaved, releasing biologically active laminin fragments. These fragments induce BTB restructuring near the basement membrane to facilitate the transit of preleptotene spermatocytes. Thus, the events of spermiation and BTB restructuring that occur simultaneously at stage VIII of the epithelial cycle can be tightly coordinated. Besides exerting the effects at the BTB, these laminin fragments also reduce the level of β1-integrins at the hemidesmosome, perturbing hemidesmosome function, which was found to further potentiate BTB restructuring [15]. Furthermore, MMP9 released by Sertoli and/or germ cells also cleaves collagen chains (e.g., collagen α3(IV), an integrin component of the basement membrane) to release the biologically active fragments to disrupt Sertoli cell TJ-barrier function. Environmental toxicants were also found to down-regulate both influx and efflux drug transporters [77], resulting in a net influx of xenobiotics into the seminiferous tubules. This model thus illustrates the likely pathways by which toxicants induce testicular injury by perturbing the apical ES-BTB-basement membrane axis, leading to toxicant-induced male infertility.
In short, the model shown in Figures 2 and 3 provides a new conception for the molecular mechanism by which toxicants induce testicular injury. This model also provides new insights regarding the vulnerability of the testis to environmental toxicants, such as cadmium and phthalates. It is obvious that the model by which toxicants block spermatogenesis via the apical ES-BTB-basement membrane functional axis will be updated rapidly as more data emerge in the near future. In the meantime, the model provides a new framework upon which functional experiments can be designed to probe these molecular events and design small molecules to prevent the entry of toxicants into the seminiferous epithelium. For instance, the model depicted in Figures 2 and 3 also illustrate that toxicants can mediate their effects by perturbing the endocytic vesicle-mediated protein trafficking at the apical ES and the BTB, thereby impeding cell adhesion at these sites to compromise spermatogenesis. In this context, it is of interest to note that gap junctions are recently shown to be crucial to maintain BTB dynamics during spermatogenesis [83], serving as coordinators of various junction types at the BTB [84, 85]; and they are also the target of environmental toxicants [86]. Thus, functional studies can be designed to explore if these toxicant-induced protein trafficking events and/or disruption of gap junction function can be blocked by manipulating the expression of protein(s) crucial to endocytosis or gap junction communications.
Concluding remarks and future perspectives
The “apical ES-BTB-basement membrane” axis coordinates and regulates cellular events that occur in the seminiferous epithelium during the epithelial cycle of spermatogenesis, and it is increasingly clear that toxicants, such as cadmium, bisphenol A, and adjudin, exert effects on the regulatory components of this axis, thereby perturbing the network of actin filament bundles that are specific to the testis at the ES. It is surprising that the strong adhesive strength and the exceedingly dynamic plasticity created by the ES at the Sertoli-spermatid interface (i.e., the apical ES) and the Sertoli cell-cell interface (i.e., the basal ES) via changes in their “bundled” and “de-bundled” (e.g., barbed end branching) configuration can be affected by the environmental toxicants cadmium and bisphenol A, as well as the male contraceptive adjudin. Damage caused by these toxicants perhaps cannot be prevented by interfering with physiological and/or signaling regulatory pathways in the testis since many of these signaling/regulatory molecules are commonly used by other mammalian cells and their disruption can lead to unwanted side-effects. However, these recent findings suggest an unprecedented opportunity to counteract these toxicants by blocking their entry into the basal and the adluminal compartments through alterations to the functions of drug transporters or the use of inhibitors and/or activators of actin-interacting proteins such as Eps8, the Arp2/3 complex, drebrin E and others. Recent advances in cancer biology to regulate drug transport functions and the actin cytoskeleton (e.g., to manipulate metastatic activity of cancer cells) may be helpful in designing therapeutic approaches to treat male infertility that arise from exposure to environmental toxicants. Table 1 summarizes a few selected examples of recent developments for targeting Eps8 and Arp3 in the context of cancer, in which actin-based tumor cell movement is disrupted during tumorigenesis to block metastasis. We propose that these compounds might be used, if adequately modified to reduce their toxicity, to block F-actin disruption at the ES to manage toxicant-induced male reproductive dysfunction.
Table 1.
Actin regulatory proteins are emerging targets of cancer chemotherapy*
| Target Protein | Roles in cancer | Name of drugs | Mechanism of actions | Related cancer | References |
|---|---|---|---|---|---|
|
| |||||
| Eps8 | -being phosphorylated on tyrosine residue in many human tumor cell line. | Mithramycin (Protocol ID: 120151; Phase 2) | -Down-regulates Eps8 expression | Lung cancer esophagus cancer | [108] |
| -a proto-oncogene | -anti-metastatic | ||||
| -regulates cell proliferation and tumor metastasis | |||||
|
| |||||
| Arp3 | -induces cell motility | Oleanane Triterpenoid (CDDO-methylester) (NCT00322140; Phase 1) | -Inhibits cell migration | Solid tumor and lymphoma | [109] |
| -inhibits mDia2-dependent filopodia formation | |||||
This Table is not intended to be exhaustive, however, it illustrates two ongoing clinical trials by targeting the two actin regulatory proteins Eps8 or Arp3 in cancer cells to correct pathological conditions in which tumor cells perturb the normal functioning of these two proteins, analogous to male reproductive dysfunction induced by environmental toxicants via their effects on the apical ES-BTB-basement membrane axis wherein Eps8 and Arp3 are being involved and disrupted, see text for details.
Box 1: Blood-testis barrier (BTB) Basal ectoplasmic specialization [basal ES, a testis-specific actin-based adherens junction (AJ)] that coexist with TJs, and gap junctions (GJs), which together with desmosome between adjacent Sertoli cells near the basement membrane constitute the BTB [21, 42, 43]. In short, the BTB is composed of multiple junction types besides the TJ. In the rat, the BTB is assembled at ~15–17 dpp (days postpartum) and it becomes fully functional by ~25 dpp [87]; the BTB is fully function in men after puberty at ~12–13 years of age. The BTB restricts paracellular and transcellular transport of substances across the barrier and confers cell polarity [21, 42, 43], segregating the seminiferous epithelium into the basal and the adluminal compartments (Figure 1). The BTB creates a specialized microenvironment in the adluminal compartment for meiosis I and II, as well as post-meiotic spermatid development. . The BTB also plays a role in conferring the immune privilege status to the testis by segregating the adluminal compartment from the systemic circulation so antigens arising in developing spermatids, many of which are expressed transiently [88], can be “shielded” from the host immune system [89]. However, germ cells (e.g., spermatogonia, spermatogonial stem cells, preleptotene spermatocytes) that reside outside the BTB in the basal compartment are also known to express a large number of germ cell-specific antigens, many of which are oncogenes and are members of the CT antigens (cancer-testis antigens) [88], and the entire seminiferous epithelium is considered an immune privileged site. These findings challenge the notion that the BTB confers immune privilege to the testis. Recent studies have shown that immunosuppressive biomolecules released by Sertoli cells are crucial to confer the immune privilege in the testis [90–92]. Collectively, these findings support the idea that the BTB may be more important in “protecting” spermatogonia/spermatogonial stem cells residing in the basal compartment from substance(s)/biomolecule(s) that can be released from the adluminal compartment. This possibility is supported, at least in part, by recent observations that the lack of a functional BTB leads type B spermatogonia and/or early spermatocytes to fail to enter meiosis; instead they undergo apoptosis [56, 93, 94]. The most notable structural feature of the BTB versus other blood-tissue barriers is the extensive actin filament bundles that lie perpendicular to the Sertoli cell plasma membrane and are sandwiched between cisternae of endoplasmic reticulum (ER) and the Sertoli cell plasma membrane found on both sides of the adjacent Sertoli cells. These networks of actin filament bundles surrounding the TJ and GJ confer the strong adhesive strength to the BTB. Even though the BTB is one of the tightest blood-tissue barriers, it is a highly dynamic ultrastructure because preleptotene spermatocytes connected in “clones” via intercellular bridges must traverse the BTB at stage VIII of the epithelial cycle. To maintain the BTB integrity during the transit of preleptotene spermatocytes, “new” BTB is first assembled at the basal region of the transiting spermatocytes before the “old” BTB is disassembled via tightly regulated events of protein endocytosis, transcytosis and recycling [95–97].
Hightlights
Environmental toxicants, such as cadmium and phthalates, disrupt male fertility.
Toxicant-induced infertility is mediated via the apical ES-BTB-BM functional axis.
Toxicant-induced damaging effects can possibly be reduced or blocked by inhibitors.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (NICHD U54 HD029990, Project 5 to C.Y.C.; R01 HD056034 to C.Y.C.
Glossary
- Arp2/3 complex.
Actin-related protein 2/3 complex is a 7-subunit protein complex consisting of Arp2, Arp3, and the Arp2/3 complex (ARPC) subunits 1–5 that nucleates actin filament branches via barbed end branching on a pre-existing actin filament [98]. The activation of the Arp2/3 complex requires N-WASP (neuronal Wiskott-Aldrich syndrome protein), SCAR/WAVE (suppressor of cAMP receptor/WASP family verprolin homolog), and cortactin, forming a 10-subunit protein complex to initiate barbed end actin branching, creating a branched actin network [98, 99]. In short, the Arp2/3 complex works in concert with Eps8 (an actin bundling and barbed end capping protein) to confer plasticity to the ES by introducing changes in the configuration of the actin filament bundles at the ES that are necessary for spermatid movement across the seminiferous and BTB restructuring.
- Ectoplasmic specialization (ES),
The ES was first found in the testis in the 1970s and is typified by hexagonally arranged bundles of actin filaments that lie perpendicular to the Sertoli cell plasma membrane and sandwiched between the Sertoli cell membrane and cisternae of endoplasmic specialization [100, 101]. It surrounds the entire head of the developing spermatid (from step 8–19 in rats). It is designated the apical ES at the Sertoli cell-cell interface and the basal ES at the BTB. Once the apical ES appears, it is the only anchoring device between Sertoli cells and spermatids, replacing desmosomes and gap junctions, until it undergoes degeneration via extensive endocytic vesicle-mediated trafficking involving endocytosis, transcytosis and recycling, creating an ultrastructure designated as apical tubulobulbar complex (apical TBC) [102]. As such, apical TBC is a degenerating apical ES that becomes visible at late stage VII to VIII tubules to prepare the epithelium for spermiation. It is now known that these changes reflect the dynamic nature of the apical ES in which “old” apical ES proteins can be re-used to assemble “new” apical ES structures as step 8 spermatids arise from spermiogenesis. However, the ES is composed of integral membrane proteins typical not only of AJs (e.g., cadherins), but also of desmosomes (e.g., desmogleins), gap junctions (e.g., connexin 43), and focal adhesion complexes (e.g., α6β1-integrin). The apical ES also confers polarity to developing spermatid so that their heads can be pointed toward the basement membrane [60, 103] to allow the packaging of the maximal number of spermatids in the tubule. Given that the actin filament bundles are present on both sides of the two adjacent Sertoli cells at the basal ES, but only on the Sertoli cell side of the apical ES at the Sertoli-spermatid interface, the basal ES is stronger. For instance, it takes much longer for the basal ES to breakdown versus the apical ES when rats exposed to toxicants such as adjudin [56].
- Eps8,
epidermal growth factor receptor pathway substrate, is an actin barbed end capping and bundling protein, promoting the formation and the maintenance of actin filament bundles at the ES. When Eps8 is associated with IRSp53 (insulin receptor tyrosine kinase substrate p53) or Abi-1 (Abelson interacting protein-1), it serves as an actin bundling [104] or barbed end capping protein [105], respectively. Eps8 is likely working in concert with filament A, an actin cross-linking and bundling protein at the ES [106] to maintain the actin filament bundles at the ES in the mammalian testis.
- Hemidesmosome,
this is an intermediate filament-based cell-extracellular matrix anchoring junction in the testis. In the testis, hemidesmosome is found at the Sertoli-basement membrane interface. The basement membrane is a modified form of extracellular matrix in the testis [79], and its two major building blocks are type IV collagen chains and laminins [107]. the constituent proteins of hemidesmosome in the testes known to date are β1-integrin and laminin-α2 chain [15]. Perturbing the hemidesmosome function, such as by a knockdown of b1-integrin, perturbs the Sertoli cell TJ-permeability barrier function at the BTB and illustrates an intriguing functional relationship between hemidesmosome and the BTB.
- Seminiferous epithelial cycle of spermatogenesis,
or the epithelial cycle, refers to the cyclic events of spermatogenesis along the seminiferous tubule in mammalian testis and can be divided into I–XIV, I–XII, and I–VI in rats, mice and humans, respectively. These stages were originally classified based on PAS (periodic acid-Schiff's reaction) staining of the Golgi region of the spermatid head, the area where acrosome is being assembled in front of the spermatid head, representing cyclic events of spermatogenesis that proceeds from stage I through XIV in the rat testis. For instance, meiosis takes place only in stage XIV and XII in rats and mice. The duration of the epithelial cycle is 12.9, 8.6 and 16 days in rats, mice and humans, illustrating that stage I advances to stage XIV in 12.9 day in the rat testis if a specific segment of a seminiferous tubule is visualized under stereomicroscopy. These stages also represent unique association of developing germ cells with the Sertoli cell in the seminiferous epithelium to prepare for specific cellular events pertinent to spermatogenesis. For instance, step 19 spermatids line up at the luminal edge of the epithelium near the tubule lumen in stage VIII tubules in rat testes to prepare for spermiation, and BTB restructuring also takes place in this stage and these two cellular events are shown to be coordinated by the apical ES-BTB-basement membrane functional axis. Furthermore, these stages in seminiferous tubules can be easily discerned under stereomicroscopy and tubules can be classified into dark spot zone (stages II–VI), dark zone (stages VII–VIII), pale zone (stages IX–XII), and weak spot zone (XIII-I) stages for studies.
- Spermiogenesis,
a crucial cellular event that occurs immediately after meiosis in which haploid spermatids undergo a series of morphological changes marked by: (i) condensation of genetic materials which are packed in the spermatid head known as the spermatid nucleus, (ii) formation of acrosome surrounding the spermatid head, and (iii) formation and elongation of the spermatid tail which are coupled with the packaging of the mitochondria to form the mid-piece of spermatids. In rats, mice, and humans, these morphological changes divide spermatids into 19, 16, and 12 steps, respectively, largely based on changes in the shape of their heads versus elongation of their tails. For instance, step 19 spermatids are first formed in stage VII of the epithelial cycle in the rat testis, which transform into spermatozoa in late stage VIII of the cycle so that spermatozoa can be released into the tubule lumen at spermiation, and be transported to the epididymis to become fully mature spermatozoa. Also, apical ES first appears in step 8 spermatids and persist until step 19 spermatids at the spermatid-Sertoli cell interface in the rat testis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST: Authors have nothing to declare
References
- 1.Wong EWP, Cheng CY. Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol Sci. 2011;32:290–299. doi: 10.1016/j.tips.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siu ER, et al. Cadmium-induced testicular injury. Toxicol Appl Pharmacol. 2009;238:240–249. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordkap L, et al. Regional differences and temporal trends in male reproductive health disorders: semen quality may be a sensitive marker of environmental exposures. Mol Cell Endocrinol. 2012;355:221–230. doi: 10.1016/j.mce.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 4.Lucas B, et al. Signaling pathways in spermatogonial stem cells and their disruption by toxicants. Birth Defects Res C Embryo Today. 2009;87:35–42. doi: 10.1002/bdrc.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauser R. Urinary phthalate metabolites and semen quality: a review of a potential biomarker of susceptibility. Int J Androl. 2008;31:112–117. doi: 10.1111/j.1365-2605.2007.00844.x. [DOI] [PubMed] [Google Scholar]
- 6.Wong EWP, et al. Cell junctions in the testis as targets for toxicants. In: McQueen CA, editor. Comprehensive Toxicology. 2nd Edition. Vol. 11. Academic Press, Elseiver; Oxford: 2010. pp. 167–188. Reproductive and Endocrine Toxicology. Hoyer, P.B., Richburg, J.H. (Ed.) [Google Scholar]
- 7.Cheng CY, et al. Environmental toxicants and male reproductive function. Spermatogenesis. 2011;1:2–13. doi: 10.4161/spmg.1.1.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hess RA. Effects of environmental toxicants on the efferent ducts, epididymis and fertility. J Reprod Fertil Suppl. 1998;53:247–259. [PubMed] [Google Scholar]
- 9.Mruk DD, Cheng CY. Environmental contaminants. Is male reproductive helath at risk? Spermatogenesis. 2011;1:283–290. doi: 10.4161/spmg.1.4.18328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parizek J, Zahor Z. Effect of cadmium salts on testicular tissue. Nature. 1956;177:1036–1037. doi: 10.1038/1771036b0. [DOI] [PubMed] [Google Scholar]
- 11.Parizek J. Sterilization of the male by cadmium salts. J Reprod Fertil. 1960;1:294–309. [Google Scholar]
- 12.Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the “blood-testis barrier” after injection of cadmium chloride in the rat. J Endocrinol. 1970;47:81–86. doi: 10.1677/joe.0.0470081. [DOI] [PubMed] [Google Scholar]
- 13.Wong CH, et al. Blood-testis barrier dynamics are regulated by α2-macroglobulin via the c-Jun N-terminal protein kinase pathway. Endocrinology. 2005;146:1893–1908. doi: 10.1210/en.2004-1464. [DOI] [PubMed] [Google Scholar]
- 14.Wong CH, et al. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- 15.Yan HHN, et al. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:8950–8955. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nature Rev Endocrinol. 2010;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lie PPY, et al. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci USA. 2012;109:12562–12567. doi: 10.1073/pnas.1202316109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su L, et al. A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats. Nat Communs. 2012;3:1185. doi: 10.1038/ncomms2171. doi:1110.1038/ncomms2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao X, et al. c-Yes regulates cell adhesion at the apical ectoplasmic specialization-blood-testis barrier axis via its effects on protein recruitment and distribution. Am J Physiol Endocrinol Metab. 2013;304:E145–E159. doi: 10.1152/ajpendo.00422.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Donnell L, et al. Spermiation: the process of sperm release. Spermatogenesis. 2011;1:14–35. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao PL, et al. TNFα-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biol Reprod. 2009;80:581–589. doi: 10.1095/biolreprod.108.073122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao PL, et al. Mono-(2-ethylhexyl) phthalate-induced disruption of junctional complexes in the seminiferous epithelium of the rodent testis is mediated by MMP2. Biol Reprod. 2010;82:516–527. doi: 10.1095/biolreprod.109.080374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao PL, et al. Transcriptional suppression of Sertoli cell Timp2 in rodents following mono-(2-ethylhexyl) phthalate exposure is regulated by CEBPA and MYC. Biol Reprod. 2011;85:1203–1215. doi: 10.1095/biolreprod.111.093484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazaud-Guittot S. Dissecting the phthalate-induced Sertoli cell injury: the fragile balance of proteases and their inhibitors. Biol Reprod. 2011;85:1091–1093. doi: 10.1095/biolreprod.111.095976. [DOI] [PubMed] [Google Scholar]
- 26.Prozialeck WC, Lamar PC. Interaction of cadmium (Cd2+) with a 13-residue polypeptide analog of a putative calcium-binding motif of E-cadherin. Biochem Biophys Acta. 1999;1451:93–100. doi: 10.1016/s0167-4889(99)00077-4. [DOI] [PubMed] [Google Scholar]
- 27.Prozialeck W. Evidence that E-cadherin may be a target for cadmium toxicity in epithelial cells. Toxicol Appl Pharmacol. 2000;164:231–249. doi: 10.1006/taap.2000.8905. [DOI] [PubMed] [Google Scholar]
- 28.Prozialeck WC, et al. Cadmium alters the localization of N-cadherin, E-cadherin, and β–catenin in the proximal tubule epithelium. Toxicol Appl Pharmacol. 2003;189:180–195. doi: 10.1016/s0041-008x(03)00130-3. [DOI] [PubMed] [Google Scholar]
- 29.Saksena SK, Lau IF. Effects of cadmium chloride on testicular steroidogenesis and fertility of male rats. Endokrinologie. 1979;74:6–12. [PubMed] [Google Scholar]
- 30.Phelps PV, Laskey JW. Comparison of age-related changes in in vivo and in vitro measures of testicular steroidogenesis after acute cadmium exposure in the Sprague-Dawley rats. J Toxicol Environ Health. 1989;27:95–105. doi: 10.1080/15287398909531281. [DOI] [PubMed] [Google Scholar]
- 31.Nikula H, et al. Inhibition of hCG-stimulated steroidogenesis in cultured mouse Leydig tumor cells by bisphenol A and octylphenols. Toxicol Appl Pharmacol. 1999;157:166–173. doi: 10.1006/taap.1999.8674. [DOI] [PubMed] [Google Scholar]
- 32.Akingbemi BT, et al. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary lutenizing hormone secretion and decreased streroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145:592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- 33.Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1517–1535. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Donnell L, et al. Estrogen and spermatogenesis. Endocr Rev. 2001;22:289–318. doi: 10.1210/edrv.22.3.0431. [DOI] [PubMed] [Google Scholar]
- 35.Prozialeck WC, et al. Cadherins and NCAM as potential targets in metal toxicity. Toxicol Appl Pharmacol. 2002;182:255–265. doi: 10.1006/taap.2002.9422. [DOI] [PubMed] [Google Scholar]
- 36.Prozialeck WC, et al. The vascular system as a target of metal toxicity. Toxicol Sci. 2008;102:207–218. doi: 10.1093/toxsci/kfm263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prozialeck WC, Edward JR. Cell adhesion molecules in chemical-induced renal injury. Pharmacol Ther. 2007;114:74–93. doi: 10.1016/j.pharmthera.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandenberg LN, et al. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vandenberg LN, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pogribny IP, Rusyn I. Environmental toxicants, epigenetics, and cancer. Adv Exp Med Biol. 2013;754:215–232. doi: 10.1007/978-1-4419-9967-2_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeung BH, et al. Endocrine disrupting chemicals: Multiple effects on testicular signaling and spermatogenesis. Spermatogenesis. 2011;1:231–239. doi: 10.4161/spmg.1.3.18019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franca LR, et al. Blood-tissue barriers: Morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol. 2012;763:237–259. [PubMed] [Google Scholar]
- 43.Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem. 2011;46:49–127. doi: 10.1016/j.proghi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Vogl AW, et al. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- 45.Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol. 1985;94:177–211. doi: 10.1016/s0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- 46.Wong EWP, et al. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem Biophys Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogl A, et al. Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol. 2000;63:1–15. doi: 10.1679/aohc.63.1. [DOI] [PubMed] [Google Scholar]
- 48.Wolski KM, et al. Strength measurement of the Sertoli-spermatid junctional complex. J Androl. 2005;26:354–359. doi: 10.2164/jandrol.04142. [DOI] [PubMed] [Google Scholar]
- 49.Green KJ, et al. Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb Perspect Biol. 2010;2:a000125. doi: 10.1101/cshperspect.a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomason HA, et al. Desmosomes: adhesive strength and signalling in health and disease. Biochem J. 2010;429:419–433. doi: 10.1042/BJ20100567. [DOI] [PubMed] [Google Scholar]
- 51.Hew KW, et al. Cadmium in vivo causes disruption of tight junction-associated microfilaments in rat Sertoli cells. Biol Reprod. 1993;49:840–849. doi: 10.1095/biolreprod49.4.840. [DOI] [PubMed] [Google Scholar]
- 52.Wiebe J, et al. Glycerol disrupts tight junction-associated actin microfilaments, occludin, and microtubules in Sertoli cells. J Androl. 2000;21:625–635. [PubMed] [Google Scholar]
- 53.Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2004;70:945–964. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- 54.Su L, et al. A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats. Nat Communs. 2012;3:1185. doi: 10.1038/ncomms2171. DOI:10.1038/ncomms2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan HHN, et al. Ectoplasmic specialization: a friend or a foe of spermatogenesis? BioEssays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mok KW, et al. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int J Androl. 2012;35:86–101. doi: 10.1111/j.1365-2605.2011.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su L, et al. Adjudin-mediated Sertoli-germ cell junction disassembly affects Sertoli cell barrier function in vitro and in vivo. Int J Biochem Cell Biol. 2010;42:1864–1875. doi: 10.1016/j.biocel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lie PPY, et al. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010;107:11411–11416. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lie PPY, et al. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–2567. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong EWP, et al. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:9657–9662. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng CY, et al. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: A review of recent data. Contraception. 2005;72:251–261. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 62.Mruk DD, et al. Anchoring junctions as drug targets: Role in contraceptive development. Pharmacol Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mruk DD, Cheng CY. Testin and actin are key molecular targets of adjudin, an anti-spermatogenic agent, in the testis. Spermatogenesis. 2011;1:137–146. doi: 10.4161/spmg.1.2.16449. doi:110.4161/spmg.4161.4162.16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng CY, et al. Adjudin disrupts spermatogenesis via the action of some unlikely partners: Eps8, Arp2/3 complex, drebrin E, PAR6 and 14-3-3. Spermatogenesis. 2011;1:291–297. doi: 10.4161/spmg.1.4.18393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li MWM, et al. Actin binding protein drebrin E is involved in junction dynamics during spermatogenesis. Spermatogenesis. 2011;1:123–136. doi: 10.4161/spmg.1.2.16393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng CY, Mruk DD. Actin binding proteins and spermatogenesis. Some unexpected findings. Spermatogenesis. 2011;1:99–104. doi: 10.4161/spmg.1.2.16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boutros T, et al. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: Roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 68.Siu MKY, et al. Adhering junction dynamics in the testis are regulated by an interplay of β-integrin and focal adhesion complex (FAC)-associated proteins. Endocrinology. 2003;144:2141–2163. doi: 10.1210/en.2002-221035. [DOI] [PubMed] [Google Scholar]
- 69.Beardsley A, et al. A complex containing α6β1-integrin and phosphorylated focal adhesion kinase between Sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J Endocrinol. 2006;190:759–770. doi: 10.1677/joe.1.06867. [DOI] [PubMed] [Google Scholar]
- 70.Li MWM, et al. Actin binding protein drebrin E is involved in junction dynamics during spermatogenesis. Spermatogenesis. 2011;1:123–136. doi: 10.4161/spmg.1.2.16393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siu ER, et al. An occludin-focal adhesion kinase protein complex at the blood-testis barrier: a study using the cadmium model. Endocrinology. 2009;150:3336–3344. doi: 10.1210/en.2008-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Su L, et al. Drug transporters, the blood-testis barrier and spermatogenesis. J Endocrinol. 2011;208:207–223. doi: 10.1677/JOE-10-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su L, et al. Drug transporter, P-glycoprotein (MDR1), is an integrated component of the mammalian blood-testis barrier. Int J Biochem Cell Biol. 2009;41:2578–2587. doi: 10.1016/j.biocel.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Setchell BP. Blood-testis barrier, functional and transport proteins and spermatogenesis. Adv Exp Med Biol. 2008;636:212–233. doi: 10.1007/978-0-387-09597-4_12. [DOI] [PubMed] [Google Scholar]
- 75.Su L, et al. P-glycoprotein regulates blood-testis barrier dynamics via its effects on the occludin/zonula occludens 1 (ZO-1) protein complex mediated by focal adhesion kinase (FAK) Proc Natl Acad Sci USA. 2011;108:19623–19628. doi: 10.1073/pnas.1111414108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mruk DD, et al. Emerging role for drug transporters at the blood-testis barrier. Trends Pharmacol Sci. 2011;32:99–106. doi: 10.1016/j.tips.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Su L, et al. Regulation of drug transporters in the testis by environmental toxicant cadmium, steroids and cytokines. Spermatogenesis. 2012;2:285–293. doi: 10.4161/spmg.22536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Su L, et al. Role of P-glycoprotein at the blood-testis barrier on adjudin distribution in the testis. A revisit of recent data. Adv Exp Med Biol. 2012;763:318–333. doi: 10.1007/978-1-4614-4711-5_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dym M. Basement membrane regulation of Sertoli cells. Endocr Rev. 1994;15:102–115. doi: 10.1210/edrv-15-1-102. [DOI] [PubMed] [Google Scholar]
- 80.Yan HHN, Cheng CY. Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem. 2006;281:17286–17303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]
- 81.Grima J, et al. Testin is tightly associated with testicular cell membrane upon its secretion by Sertoli cells whose steady-state mRNA level in the testis correlates with the turnover and integrity of inter-testicular cell junctions. J Biol Chem. 1997;272:6499–6509. doi: 10.1074/jbc.272.10.6499. [DOI] [PubMed] [Google Scholar]
- 82.Siu MKY, et al. The interplay of collagen IV, tumor necrosis factor-α gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloprotease-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- 83.Pointis G, et al. Physiological and physiopathological aspects of connexins and communicating gap junctions in spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1607–1620. doi: 10.1098/rstb.2009.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li MWM, et al. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci USA. 2009;106:10213–10218. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li MWM, et al. Connexin 43 is critical to maintain the homeostasis of blood-testis barrier via its effects on tight junction reassembly. Proc Natl Acad Sci USA. 2010;107:17998–18003. doi: 10.1073/pnas.1007047107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pointis G, et al. Testicular connexin 43, a precocious molecular target for the effect of environmental toxicants on male fertility. Spermatogenesis. 2011;1:303–317. doi: 10.4161/spmg.1.4.18392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mok KW, et al. A study to assess the assembly of a functional blood-testis barrier in developing rat testes. Spermatogenesis. 2011;1:270–280. doi: 10.4161/spmg.1.3.17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cheng YH, et al. Cancer/testis (CT) antigens, carcinogenesis and spermatogenesis. Spermatogenesis. 2011;1:209–220. doi: 10.4161/spmg.1.3.17990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fijak M, Meinhardt A. The testis in immune privilege. Immunol Rev. 2006;213:66–81. doi: 10.1111/j.1600-065X.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 90.Doyle TJ, et al. Immunoprotective properties of primary Sertoli cells in mice: potential functional pathways that confer immune privilege. Biol Reprod. 2012;86:1–14. doi: 10.1095/biolreprod.110.089425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol. 2011;335:60–68. doi: 10.1016/j.mce.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 92.Kaur G, et al. Genetically engineered immune privileged Sertoli cells - a new road to cell based gene therapy. Spermatogenesis. 2012;2:23–31. doi: 10.4161/spmg.19119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Toyama Y, et al. Neonatally administered diethylstilbestrol retards the development of the blood-testis barrier in the rat. J Androl. 2001;22:413–423. [PubMed] [Google Scholar]
- 94.Kopera IA, et al. An in vivo study on adjudin and blood-testis barrier dynamics. Endocrinology. 2009;150:4724–4733. doi: 10.1210/en.2008-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yan HHN, et al. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Su L, et al. Differential effects of testosterone and TGF-β3 on endocytic vesicle-mediated protein trafficking events at the blood-testis barrier. Exp Cell Res. 2010;316:2945–2960. doi: 10.1016/j.yexcr.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wong EWP, et al. Regulation of blood-testis barrier dynamics by TGF-β3 is a Cdc42-dependent protein trafficking event. Proc Natl Acad Sci USA. 2010;107:11399–11404. doi: 10.1073/pnas.1001077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dos Remedios CG, et al. Actin binding proteins: Regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83:433–473. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- 99.Lie PPY, et al. Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1581–1592. doi: 10.1098/rstb.2009.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fawcett D. Ultrastructure and function of the Sertoli cell. In: Hamilton D, Greep R, editors. Handbook of Physiology. American Physiological Society; 1975. pp. 21–25. [Google Scholar]
- 101.Russell LD. Observations on rat Sertoli ectoplasmic ('junctional') specializations in their association with germ cells of the rat testis. Tissue Cell. 1977;9:475–498. doi: 10.1016/0040-8166(77)90007-6. [DOI] [PubMed] [Google Scholar]
- 102.Russell LD. Spermatid-Sertoli tubulobulbar complexes as devices for elimination of cytoplasm from the head region in late spermatids of the rat. Anat Rec. 1979;194:233–246. doi: 10.1002/ar.1091940205. [DOI] [PubMed] [Google Scholar]
- 103.Wong EWP, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol. 2009;278:309–353. doi: 10.1016/S1937-6448(09)78007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Disanza A, et al. Regulation of cell shape by Cdc42 is mediated by the snergic actin-bundling activity of Eps8-IRSp53 complex. Nature Cell Biol. 2006;8:1337–1347. doi: 10.1038/ncb1502. [DOI] [PubMed] [Google Scholar]
- 105.Disanza A, et al. Eps8 controls actin-based motility by capping the barbed ends of actin filaments. Nature Cell Biol. 2004;6:1180–1188. doi: 10.1038/ncb1199. [DOI] [PubMed] [Google Scholar]
- 106.Su WH, et al. Filamin A is a regulator of blood-testis barrier assembly during postnatal development in the rat testis. Endocrinology. 2012;153:5023–5035. doi: 10.1210/en.2012-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siu MKY, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. BioEssays. 2004;26:978–992. doi: 10.1002/bies.20099. [DOI] [PubMed] [Google Scholar]
- 108.Yang TP, et al. Mithramycin inhibits human epithelial carcinoma cell proliferation and migration involving downregulation of Eps8 expression. Chem Biol Interact. 2010;183:181–186. doi: 10.1016/j.cbi.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 109.To C, et al. Synthetic triterpenoids target the Arp2/3 complex and inhibit branched actin polymerization. J Biol Chem. 2010;285:27944–27957. doi: 10.1074/jbc.M110.103036. [DOI] [PMC free article] [PubMed] [Google Scholar]