Abstract
Selenoproteins are present in all three domains of life and are responsible for a major part of a cell’s antioxidant defense against reactive oxygen species. Synthesis of selenoproteins requires the decoding of a UGA codon as selenocysteine (Sec) instead of translation termination. Sec is incorporated into the growing polypeptide chain during translation elongation and is known to require a set of highly specific factors: The Sec insertion sequence (SECIS) element in the 3′ untranslated region (3′ UTR), Sec-tRNASec, the Sec-specific elongation factor eEFSec, and SECIS binding protein 2 (SBP2). Since reconstitution has not been reported, whether these factors are sufficient is unknown. Here we report a novel in vitro translation system in which Sec incorporation has been reconstituted from purified components introduced into a Sec naive system. In addition, we developed a novel method to purify Sec-tRNASec and active eEFSec/GTP/tRNA ternary complex. We found that the known basal factors are sufficient for Sec incorporation in vitro. Using this highly manipulable system, we have also found that ribosomes from non-Sec utilizing organisms cannot support Sec incorporation and that some SECIS elements are intrinsically less efficient than others. Having identified the essential set of factors, this work removes a significant barrier to our understanding of the mechanism of Sec incorporation.
Keywords: ribosome, translation elongation, selenocysteine, SECIS
Introduction
Selenium is cotranslationally incorporated as the 21st amino acid selenocysteine (Sec) into selenoproteins in all three domains of life, although their expression is notably lacking in higher plants and fungi. There are at least 25 selenoproteins in humans and their functions range from reduction of oxidized phospholipids to protein folding1. Deletion of Sec-tRNASec is embryonic lethal in mice, thus accentuating the biological importance of Sec incorporation2. During translation of selenoprotein mRNAs, Sec is encoded by an in-frame UGA codon, which is normally a stop codon. To date, it is known that this recoding event requires three trans-acting factors: 1) Sec-tRNASec, 2) Sec-specific elongation factor eEFSec, and the 3) SECIS binding protein 2 (SBP2) as well as one cis-acting RNA sequence, the Sec insertion sequence (SECIS) element in the 3′ untranslated region (3′ UTR). While other factors have been suggested to be involved in the Sec incorporation process, only these four factors have been shown to be required, although they have never been shown to be sufficient.
Eukaryotic SECIS elements are stable stem-loop structures that belong to the kink-turn family of RNA structures. They are required for Sec incorporation, and are exclusively found in the 3′ UTR. They are comprised of two helices separated by an internal loop of 4–18 nucleotides3, and they have two conserved regions: the SECIS core (RUGA, where R=A or G) and an apical AAR motif. The SECIS core forms the base of helix 2, which contains four non-Watson-Crick base pairs, the tandem G.A/A.G “quartet” and a 5′ RU4. The core is also the binding site for SBP2 and this binding is essential for Sec incorporation5,6. SECIS elements also have an apical AAR motif usually comprised of three unpaired adenosines that are required for Sec incorporation7, but the function of this AAR motif is still unknown. SECIS-specific regulation of selenoprotein expression has been studied before using all 26 human SECIS elements8. These studies suggested that the SECIS elements differ in their UGA recoding efficiency, but whether the differences were due to intrinsic properties of the SECIS elements or the function of an unknown protein factor was not determined.
SBP2 was first detected as a protein that specifically cross-linked to the GPX4 3′UTR with a wild-type SECIS9 and was subsequently shown to be required for Sec incorporation5. SBP2 specifically binds to the SECIS core, and its binding is not influenced by mutations in the conserved AAR motif of the SECIS element6,9. It was subsequently shown that mammalian SBP2 consists of three domains: an N-terminal domain that is dispensable for Sec incorporation in vitro, a central Sec incorporation domain (SID) that is required for Sec incorporation and wild type levels of SECIS binding, and the C-terminal RNA binding domain (RBD), which contains a canonical L7Ae RNA binding domain that is required for SECIS binding10,11. Studies of structure-function relationships within SBP2 have been greatly aided by the rabbit reticulocyte lysate (RRL) in vitro translation system, which is replete with all Sec incorporation factors except SBP2.
A specialized translation elongation factor, eEFSec, is required for Sec incorporation in eukaryotes
Identification of the eukaryotic Sec specific translation elongation factor by homology to EF-Tu, eEF1A, and archaeal SelB was reported independently by two groups12,13. eEFSec is a G-protein that binds GTP and GDP with similar affinity, and thus it likely does not require guanine exchange factor (GEF)12,13. The same studies have also demonstrated that eEFSec specifically binds Sec-tRNASec but not its precursor, Ser-tRNASec. eEFSec has four domains and based on sequence conservation, the first three domains of eEFSec are similar to the canonical eukaryotic translation elongation factor eEF1A, but it has a C-terminal extension termed Domain IV. Recently, it has been shown that this domain is required for Sec incorporation and is involved in Sec-tRNASec binding, GTPase regulation and interactions with SBP2 in a SECIS-dependent manner14. This study employed a partially reconstituted in vitro translation system that was limiting for eEFSec, thus allowing the study of eEFSec-dependent Sec incorporation to show Domain IV is required for all of the known functions for eEFSec: Sec-tRNASec binding, GTP hydrolysis and Sec incorporation14.
Mechanistically, it is has been presumed that SBP2 and the SECIS element provide the specificity needed for decoding only select in-frame UGA codons by the eEFSec ternary complex, thus preventing translation termination. Since Sec incorporation has not been reconstituted in vitro, the exact mechanism of this specificity still remains unclear. In this study we have created a novel in vitro translation system in which Sec incorporation was reconstituted from purified components added to a Sec-naive wheat germ in vitro translation lysate. Using this system, we report three major findings: 1) the known core factors are sufficient for Sec incorporation, 2) ribosomes from non-Sec utilizing species cannot support Sec incorporation and 3) SECIS elements possess intrinsically different capacities to promote Sec incorporation in vitro.
Results and Discussion
A functional assay to determine minimum requirements for Sec incorporation
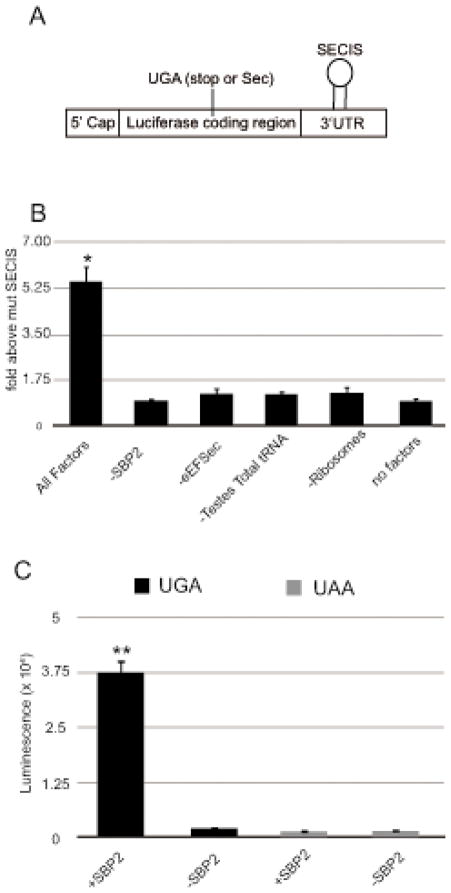
One of the major hurdles in determining the core mechanism of Sec incorporation is creating a system in which Sec incorporation can be reconstituted from purified factors. A relatively small set of trans- and cis- acting factors is known to be required for Sec incorporation, but whether these factors are sufficient is unknown. Higher plants do not utilize Sec and they do not possess any of the factors required for selenoprotein synthesis20. They are, therefore, a potentially useful system to study reconstitution and modification of the Sec incorporation pathway. In an attempt to reconstitute Sec incorporation in vitro, we used wheat germ lysate as a source of translation factors and energy required to support the reaction. To this lysate we added purified recombinant Sec incorporation factors including the fully active Xpress-His tagged C-terminal fragment of SBP2 (XH-CTSBP2), FLAG tagged eEFSec (FLAG-eEFSec), total aminoacyl tRNA (aa-tRNA) from rat testis (which is a rich source of Sec-tRNASec) and mammalian ribosomes. Sec incorporation was monitored with a luciferase reporter consisting of the luciferase coding region with a single in-frame UGA codon at position 258 followed by the rat GPX4 SECIS element (Figure 1A). This assay is a sensitive measure of Sec incorporation since the production of full length and active luciferase is strictly SECIS and SBP2-dependent as demonstrated by the lack of luciferase activity when mutant SECIS elements lacking the conserved AUGA motif are used16. As shown in Figure 1B (lane 1), the addition of the Sec incorporation factors described above was sufficient to observe luciferase activity that was about 6-fold higher than the background obtained from the mRNA with a mutant SECIS element. Figure 1B also shows that reactions lacking any one or all of the components were devoid of Sec incorporation activity (lanes 2–6). This data clearly shows that the known factors are sufficient for Sec incorporation in vitro. To further verify that the incorporation event was selenocysteine, we tested a luciferase construct possessing a UAA stop codon at position 258. In this case, no luciferase activity above the control with a mutant SECIS element was observed (Figure 1C). Considering that an unknown factor may be carried in our ribosome preparation, we gradient purified ribosomal subunits and found a similar level of activity (~6-fold above background), making it unlikely that the ribosomes are a source of an unidentified essential factor (data not shown). It is clear from previous studies that there is a complex interaction between trans and cis-acting factors during Sec incorporation. This novel assay can be used to study the function and dynamics of each of the factors involved and also it will enable the screening of for cis- and trans- acting enhancers or suppressors of Sec incorporation.
Figure 1.
Reconstitution of Sec incorporation in wheat germ in vitro translation lysate. In vitro translation of the Sec incorporation reporter mRNA (A) in 50 % of wheat germ lysate in the presence or absence of 160 nM XH-SBP2 and FLAG-eEFSec recombinant proteins, 1.25 μg of total testes aminoacylated tRNA and 80 nM of salt washed rabbit ribosomes (B). Data was normalized for luciferase activity from mutant GPX4 SECIS element. The data represents the mean and standard deviation of three independent experiments (n=3). The asterisk (*) denotes a significant difference vs. no factors by student’s t-test (p < 0.02). (C) In vitro translation of a Sec incorporation reporter mRNA that has a UAA codon instead of the UGA codon shown in (A). Raw luciferase activity (luminescence) was measured by luminometry. The double asterisk (**) denotes a significant difference vs. no SBP2 by student’s t-test (p < 0.02).
Purification of Sec-tRNASec
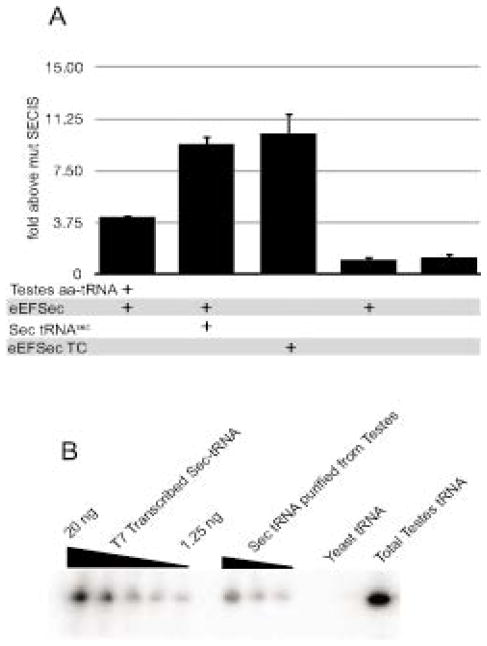
The current method of purification for Sec-tRNASec is labor intensive and requires the use of three different columns, one of which is not commercially available21,22. This makes Sec-tRNASec a limiting reagent in the field of selenium biology, and there is thus a need for a simple and fast method for purification of Sec-tRNASec. Since it has been previously shown that eEFSec specifically binds Sec-tRNASec12,13, we decided to perform affinity purification using recombinant FLAG-eEFSec, which should selectively bind Sec-tRNASec from a pool of total aa-tRNAs extracted from rat testis. For this, anti FLAG magnetic beads were incubated with FLAG-eEFSec for an hour following which total rat testis aa-tRNA and GTP were added and incubated for another hour. Finally, the eEFSec/GTP/tRNA ternary complex was eluted with FLAG peptide and part of the resulting eluate was used directly for a Sec incorporation assay and the other part was used to extract Sec-tRNASec. As shown in Figure 2A, we observed Sec incorporation that was ~9.5 fold above the mutant SECIS background upon addition of the purified Sec-tRNASec obtained from the eEFSec pulldown (lane 2) as well as from the eEFSec/GTP/tRNA ternary complex (lane 3). Both of these sources of Sec-tRNASec showed greater activity than the addition of total testis aa-tRNA (lane 1). Sec incorporation was not observed in reactions lacking added Sec-tRNASec or FLAG-eEFSec (lanes 4–5). The presence of Sec-tRNASec was confirmed and quantified by Northern blot, and based on this method of detection, we have estimated a purification of approximately 35-fold (Figure 2B). Together these results demonstrate that we have developed a method to obtain highly enriched and functional Sec-tRNASec and also we have isolated an active eEFSec/GTP/tRNA ternary complex. This is the first time it has been shown that eEFSec, GTP and Sec-tRNA form an active complex that is able to support Sec incorporation in vitro. One of the major mechanistic questions in Sec incorporation is how the delivery of Sec-tRNASec by eEFSec to the ribosome is regulated. Since we have established a rapid method to purify Sec-tRNASec and an assay to test its functionality, the feasibility of this type of work is greatly increased.
Figure 2.
Purification of Sec-tRNASec A) In vitro translation of the Sec incorporation reporter mRNAs as described in Figure 1 in the presence of total testis aa-tRNA, 125 ng of Sec-tRNASec, purified FLAG-eEFSec and/or purified FLAG-eEFSec/GTP/tRNA ternary complex (TC) as indicated. The data represents the mean and standard deviation of three independent experiments. B) The presence of Sec-tRNASec was confirmed and quantified by Northern blot. Two-fold serial dilution of in vitro transcribed tRNASec (1.25 – 20ng) and samples derived from tRNA purifications as indicated were analyzed by Northern analysis and hybridized to a probe complementary to the anticodon loop of tRNASec. The amount of Sec-tRNASec from each source was determined by densitometry using the in vitro transcribed tRNASec as a standard curve.
Ribosomes from non Sec-utilizing organisms are not able to support Sec incorporation
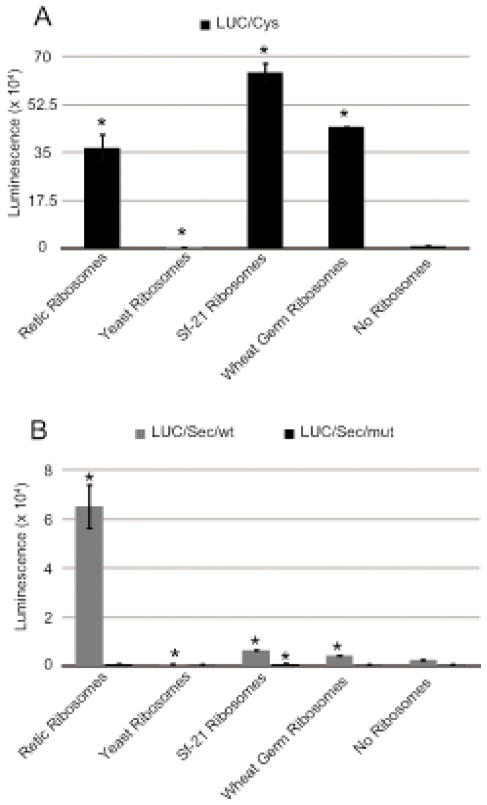
Selenoproteins are present in all three domains of life. However, the distribution of these selenoproteins among eukaryotic taxa varies greatly. For instance, there are no selenoproteins in yeast, higher plants and several insect species, including the fall armyworm, Spodoptera frugiperda. This fact allows us to ask the important question as to whether all ribosomes are intrinsically able to incorporate selenocysteine or whether specific ribosomal structures may have evolved to function in Sec-utilizing organisms. Previous work has shown that SBP2 is able to specifically modify the conformations of Helix 89 and expansion segment 31 in mammalian large subunit rRNA18. Since neither of these regions is well conserved, it is possible that Sec-specific functionality could reside at these locations. To examine this hypothesis, we tested ribosomes for Sec incorporation as well as total translation from Sec and non-Sec utilizing organisms. For this assay, rabbit reticulocyte lysate (RRL) was depleted of ribosomes by centrifugation, and the post-ribosomal supernatant was used to examine the function of exogenous ribosomes in Sec incorporation. Purified salt-washed ribosomes derived from rabbit reticulocyte lysate, Spodoptera frugiperda Sf21 cells, wheat germ lysate and Saccharomyces cerevisiae were added to the ribosome-depleted RRL. Sec incorporation was studied using the luciferase construct described in the previous section. Total translation was measured using a luciferase coding region without any in-frame UGA codon and without a SECIS element. Figure 3 shows total translation (Figure 3A) and Sec incorporation (Figure 3B) derived from ribosomes of varying origin. Notably, the ribosomes from non Sec-utilizing Spodoptera frugiperda and wheat germ can readily support total translation but only very low levels of Sec incorporation. Ribosomes from Sec-utilizing rabbit reticulocyte could support translation as well as Sec incorporation. Interestingly, however, yeast ribosomes could not support total translation or Sec incorporation. The lack of yeast ribosome activity for general translation is a surprise since they have previously been used successfully with mammalian elongation factors23. The quality of the yeast ribosomes was verified using a poly(Phe) synthesis assay (data not shown), so it is likely that one or more mammalian initiation factors are incompatible with yeast ribosomes. Although there was a small but statistically significant amount of Sec incorporation with plant and insect ribosomes, the extremely low levels of activity indicate that there is a fundamental difference at the ribosomal level between Sec- and non-Sec-utilizing species. This finding has significant implications for efforts to reconstitute Sec incorporation in genetically tractable Sec-naive organisms such as yeast, but at the same time it offers a means to potentially screen ribosomal mutant libraries for a gain of Sec incorporation activity, thus potentially permitting an unprecedented level of understanding regarding the molecular mechanism of Sec incorporation.
Figure 3.
Mammalian ribosomes are required for Sec incorporation. Total translation (A) or Sec incorporation (B) in ribosome-depleted RRL in the presence of 80 nM salt washed ribosomes from rabbit reticulocyte lysate, Saccharomyces cerevisiae, Spodoptera frugiperda Sf21 cells or wheat germ lysate as indicated. The asterisk (*) denotes a significant difference vs. no ribosomes by student’s t-test (p < 0.02).
The Role of SECIS elements on expression of selenoproteins
Previously, the UGA recoding efficiency of all human SECIS elements was analyzed in vitro using RRL. This study revealed significant differences in recoding efficiency depending on the SECIS element used8. Since RRL is only limiting for SBP2 and is replete for all the other Sec incorporation factors16, it remains unknown whether the differences in the UGA recoding efficiency can be attributed to an intrinsic property of the SECIS element or an as-yet unidentified factor. Having developed a system free of other Sec incorporation factors, we decided to study the UGA recoding efficiency of three human SECIS elements: glutathione peroxidase 4 (hGpx4), thioredoxin reductase 3 (hTrxR3), and Selenoprotein O (SelO) using our novel in vitro translation system in which Sec incorporation is dependent on externally added Sec incorporation factors. Prior work showed that use of the GPX4 SECIS element resulted in more than 4-fold greater Sec incorporation efficiency than the TrxR3 SECIS element and more than 50-fold more than the SelO SECIS element in RRL8. To test whether this difference is due to an intrinsic property of the SECIS element and its interactions with the known Sec incorporation factors or the ribosome, we used the same luciferase reporter described above but followed by the human GPX4, TrxR3 or SelO SECIS element. As a positive control we also tested these constructs in RRL. As shown in Figure 4, Sec incorporation activity of the hGPX4 SECIS element was found to be about 2-fold higher than that of hTrxR3 and about 30 or 50-fold higher than SelO in wheat germ (Figure 4A) or RRL (Figure 4B), respectively. This difference cannot be attributed to differential SBP2 binding since it was previously shown with the same SECIS elements that SBP2 binds TrxR3 (4 nM) and SelO (7 nM) with slightly higher affinity than GPX4 (13 nM)24. In addition, the difference observed here is not due to differential RNA stability since equal amounts of the protein product that resulted from termination at the UGA codon were observed when reactions were labeled with 35S Methionine (data not shown). We therefore conclude that the difference in the recoding efficiency is an intrinsic property of SECIS elements, likely at the level of direct SECIS-ribosome interactions during decoding.
Figure 4.
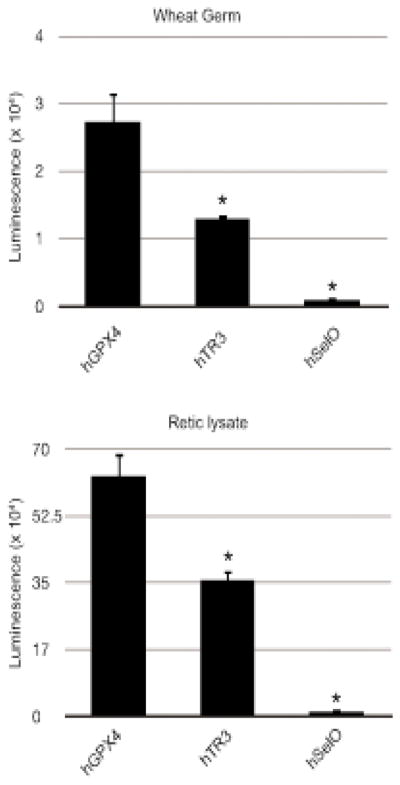
Differential SECIS element efficiency. (A) In vitro translation of the Sec incorporation reporter mRNA bearing the hGpx4, hTrxR3 and Selenoprotein O SECIS elements in wheat germ lysate with 160 nM XH-SBP2, eEFSec/GTP/tRNA ternary complex and 80 nM of salt washed rabbit ribosomes. The data represents the mean and standard deviation of three independent experiments. (B) Same as in (A) except the translation was performed in rabbit reticulocyte lysate without added ribosomes. The data represents the mean and standard deviation of three independent experiments. The asterisk (*) denotes a significant difference vs. hGPX4 by student’s t-test (p < 0.02).
Conclusion
Here we have demonstrated that no additional factors are required for the core Sec incorporation reaction. The efficiency of Sec incorporation using this in vitro translation assay was found to be 3% of total translation whereas it has been reported that the efficiency of Sec incorporation using RRL is in between 7%–10% of total translation. Although this difference is likely due to the inability of the wheat ribosomes to participate in Sec incorporation, it is also possible that there is a factor or factors that can be added to increase the efficiency of Sec incorporation. Several factors have been implicated in being involved in Sec incorporation. In one case, ribosomal protein L30 is being supplied as part of our ribosome prep, so we cannot assess its essentiality. Other factors that are not found in wheat such as SECp4325, Nucleolin26, and the GTPase-activating protein GAPsec27, can be tested in this system for specific roles in the core Sec incorporation reaction. Indeed, one of the most important aspects of this work is having established a system in which the individual functions of these and other as yet undiscovered regulatory factors can be tested in a quantitative and controlled fashion.
Materials and Methods
Recombinant protein expression and purification
Recombinant SBP2 was purified as described previously15. FLAG-eEFSec was purified using anti-FLAG M2 magnetic beads (Sigma-Aldrich) as previously described14. Quantitation of the recombinant proteins was performed on SDS-PAGE gels using an ovalbumin standard curve.
Extraction of total aminoacylated tRNA from testes extract
Fresh trimmed rat testes were purchased from Pel Freeze (St. Louise, MO). The tissue was homogenized using a hand blender in translation buffer (20 mM Tris-HCl, 100 mM KCl, 2.5 mM MgCl2, 2 mM DTT, 0.4 mM GTP, 0.25 mM spermidine, 20% glycerol and Roche EDTA-free protease inhibitors). Crude extract was centrifuged at 12,000 x g for 30 min at 4 C. Supernatant was collected and used for extraction of aminoacylated tRNA. To 20 ml of the supernatant, 12 ml of H2O, 8 ml of 5X Buffer T (50 mM NaOAc, 3.25 M NaCl, 50 mM MgCl2, 5 mM EDTA) and 40 ml of phenol pH 4 was added. The solution was briefly vortexed and centrifuged at 12000 x g for 10 mins at 40C. The aqueous phase was transferred to another tube and re-extracted with one volume of phenol pH 4 to remove remaining protein contamination. RNA was precipitated with 2.5 volumes of 100% ethanol and stored at −800C for 10 min. RNA was pelleted at 12,000 x g for 15 min at 40C and resuspended in 1.6 ml of 1X Buffer T. RNA was re-pelleted by ethanol precipitation, washed once with 70% ethanol and air-dried for 10 min. Pellet was resuspended in H2O. To verify that aminoacyl tRNA was recovered, an aliquot of the purified tRNA was deacylated in Buffer N (25 mM Tris, pH 9) at 37 C for 30 min. This deacylated tRNA did not support Sec incorporation or eEFSec binding (data not shown).
In vitro reconstitution of Sec incorporation
Sec incorporation activity was measured with a luciferase mRNA reporter containing a UGA-Sec codon at position 258 of the coding region and the rat GPX4 SECIS element in the 3′ UTR16. Non-specific read-through activity was measured using a similar luciferase mRNA reporter but the AUGA core (SBP2 binding site) in the SECIS element was deleted. All RNAs have an encoded 100 nt poly(A) tail, which is not required for in vitro translation or Sec incorporation17, except for those used in Figures 1C and 4. A typical reaction contained 6.5 μl of wheat germ extract, 100 ng of luciferase mRNA reporter, 160 nM of SBP2 and eEFSec recombinant proteins, 1.25 μg of total testes aminoacylated tRNA and 80 nM of salt washed ribosomes from rabbit reticulocyte lysate. Sec incorporation and read-through reactions were incubated at 30 C for 1 hour and measured for luminescence in a 96-well plate luminometer (Berthold Tristar).
Purification of Sec-tRNASec
Two liters of bacterial culture over-expressing Flag-eEFSec was pelleted and resuspended in 40 ml of Buffer A (20 mM Tris-HCl pH 7.5, 20 mM KCl, 0.1 mM EDTA, 25% Glycerol, 500 mM NaCl, 1% Tween, and 0.5 mM PMSF). Solutions were sonicated four times at 2 sec/ml with 1–2 min resting periods on ice and then centrifuged at 15,000 x g for 15 min at 4°C. Next, 1 ml of anti-FLAG M2 magnetic beads (Sigma-Aldrich) was incubated with a total of 80 ml of protein extract, in 40 ml aliquots, for 2 h each at 4°C. After the binding step, the beads were washed 5 times with Buffer A without PMSF followed by 5 times with Buffer B (20 mM Tris-HCl pH 7.5, 20 mM KCl, 0.1 mM EDTA, 25% Glycerol). To obtain pure Sec-tRNASec 7.5 mg of total testes aa-tRNA and 0.5 mM GTP were added to the washed beads and incubated at 4°C for 1 h. Protein was eluted in 200 μl of Buffer B with 250 μg/ml of 3X FLAG peptide for 30 min at 4°C. Half of the eluent was used for Sec incorporation assays and the other half was used for tRNA extraction as described in the previous section. The tRNA obtained after extraction was used for the Sec incorporation assay and Northern analysis.
Northern analysis
Total tRNA extracted from testis, purified Sec-tRNASec and T7 polymerase transcribed Sec-tRNASec were loaded onto gels and then electroblotted onto a nylon membrane. The membrane was hybridized with 32P-labeled Sec-tRNA probe using ULTRAhyb-Oligo (Ambicon) solution and conditions. The washed membrane was exposed to a PhosphorImager screen and radioactive signal quantitated using IMAGEQuant software. The probe sequence is: CAGCTACAGGTTTGAAGCCTGCACC.
Purification and assay of Ribosomes
80S ribosomes from RRL, wheat germ, and Sf-21 lysate (Promega) were purified as described previously18. Purification of 40 and 60S subunits from RRL was performed as reported earlier19. To deplete ribosomes, RRL was centrifuged at 300,000 x g for 1 hr at 4 C. The supernatant was removed and centrifuged again for 1 hr at 4 C. The supernatant following the second centrifugation was used for the assay. Total translation was measured using a wild-type luciferase reporter16. Sec incorporation activity and non-specific read-through activity were measured using the same constructs as described in the previous section. 12.5 μl reactions contained 8 μl of ribosome-depleted RRL,100 ng of luciferase mRNA reporter, 160 nM of SBP2 and 80 nM of salt washed ribosomes from rabbit reticulocyte lysate. Total translation, Sec incorporation and read-through reactions were incubated at 30 C for 1 hr and measured for luminescence in a 96-well plate luminometer (Berthold Tristar).
Constructs and in vitro translation to test the role of SECIS elements
The luciferase reporter construct with a UGA codon at position 258 and rat GPX4 SECIS has been previously described16. The human GPX4, TrxR3 and Sel O SECIS constructs were subcloned as Pac I/Not I restriction fragment into the luciferase reporter plasmid. The reporter plasmids bearing the human GPX4, TrxR3 and Sel O SECIS elements were linearized with Not I and used as templates to transcribe capped mRNAs with the T7 mMessage/mMachine kit (Ambion) according to the manufacturer’s protocol. Both the in vitro transcribed mRNAs were used in Sec incorporation assay described in the previous section as well as in RRL described previously16.
Highlights.
Sec incorporation requires three trans-acting factors: 1) Sec-tRNASec, 2) Sec-specific elongation factor eEFSec, and the 3) SECIS binding protein 2 (SBP2) and one cis-acting RNA sequence, the Sec insertion sequence (SECIS) element in the 3′ untranslated region (3′ UTR).
We show that these core factors are sufficient via in vitro reconstitution of Sec incorporation and also prove that ribosomes from non-Sec utilizing species cannot support Sec incorporation.
This assay is used to demonstrate that SECIS elements possess intrinsically different capacities to promote Sec incorporation in vitro.
In addition we have developed a novel method to purify Sec-tRNASec using anti-FLAG magnetic beads and recombinant FLAG-eEFSec.
Acknowledgments
We thank Arjun Sasikumar for help with ribosomes and critical discussions. This work was supported by PHS grants GM094833 and GM077073 (P.R.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Papp LV, Holmgren A, Khanna KK. Selenium and selenoproteins in health and disease. Antioxid Redox Signal. 2010;12:793–795. doi: 10.1089/ars.2009.2973. [DOI] [PubMed] [Google Scholar]
- 2.Bösl MR, Takaku K, Oshima M, Nishimura S, Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp) Proc Natl Acad Sci U S A. 1997;94:5531–5534. doi: 10.1073/pnas.94.11.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapple CE, Guigó R, Krol A. SECISaln, a web-based tool for the creation of structure-based alignments of eukaryotic SECIS elements. Bioinformatics. 2009;25:674–675. doi: 10.1093/bioinformatics/btp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fagegaltier D, Lescure A, Walczak R, Carbon P, Krol A. Structural analysis of new local features in SECIS RNA hairpins. Nucleic Acids Res. 2000;28:2679–2689. doi: 10.1093/nar/28.14.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Copeland PR, Fletcher JE, Carlson BA, Hatfield DL, Driscoll DM. A novel RNA binding protein, SBP2, is required for the translation of mammalian selenoprotein mRNAs. EMBO J. 2000;19:306–314. doi: 10.1093/emboj/19.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Copeland PR, Driscoll DM. Purification, redox sensitivity, and RNA binding properties of SECIS-binding protein 2, a protein involved in selenoprotein biosynthesis. J Biol Chem. 1999;274:25447–25454. doi: 10.1074/jbc.274.36.25447. [DOI] [PubMed] [Google Scholar]
- 7.Berry MJ, Banu L, Harney JW, Larsen PR. Functional characterization of the eukaryotic SECIS elements which direct selenocysteine insertion at UGA codons. EMBO J. 1993;12:3315–3322. doi: 10.1002/j.1460-2075.1993.tb06001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latrèche L, Jean-Jean O, Driscoll DM, Chavatte L. Novel structural determinants in human SECIS elements modulate the translational recoding of UGA as selenocysteine. Nucleic Acids Res. 2009;37:5868–5880. doi: 10.1093/nar/gkp635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lesoon A, Mehta A, Singh R, Chisolm GM, Driscoll DM. An RNA-binding protein recognizes a mammalian selenocysteine insertion sequence element required for cotranslational incorporation of selenocysteine. Mol Cell Biol. 1997;17:1977–1985. doi: 10.1128/mcb.17.4.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Copeland PR, Stepanik VA, Driscoll DM. Insight into mammalian selenocysteine insertion: domain structure and ribosome binding properties of Sec insertion sequence binding protein 2. Mol Cell Biol. 2001;21:1491–1498. doi: 10.1128/MCB.21.5.1491-1498.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donovan J, Caban K, Ranaweera R, Gonzales-Flores JN, Copeland PR. A novel protein domain induces high affinity selenocysteine insertion sequence binding and elongation factor recruitment. J Biol Chem. 2008;283:35129–35139. doi: 10.1074/jbc.M806008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fagegaltier D, Hubert N, Yamada K, Mizutani T, Carbon P, Krol A. Characterization of mSelB, a novel mammalian elongation factor for selenoprotein translation. EMBO J. 2000;19:4796–4805. doi: 10.1093/emboj/19.17.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tujebajeva RM, Copeland PR, Xu XM, Carlson BA, Harney JW, Driscoll DM, Hatfield DL, Berry MJ. Decoding apparatus for eukaryotic selenocysteine insertion. EMBO Rep. 2000;1:158–163. doi: 10.1093/embo-reports/kvd033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Flores JN, Gupta N, Demong LW, Copeland PR. The selenocysteine-specific elongation factor contains a novel and multi-functional domain. J Biol Chem. 2012;287:38936–38945. doi: 10.1074/jbc.M112.415463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinzy SA, Caban K, Copeland PR. Characterization of the SECIS binding protein 2 complex required for the co-translational insertion of selenocysteine in mammals. Nucleic Acids Res. 2005;33:5172–5180. doi: 10.1093/nar/gki826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta A, Rebsch CM, Kinzy SA, Fletcher JE, Copeland PR. Efficiency of mammalian selenocysteine incorporation. J Biol Chem. 2004;279:37852–37859. doi: 10.1074/jbc.M404639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donovan J, Copeland PR. The efficiency of selenocysteine incorporation is regulated by translation initiation factors. J Mol Biol. 2010;400:659–664. doi: 10.1016/j.jmb.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caban K, Copeland PR. Selenocysteine insertion sequence (SECIS)-binding protein 2 alters conformational dynamics of residues involved in tRNA accommodation in 80 S ribosomes. J Biol Chem. 2012;287:10664–10673. doi: 10.1074/jbc.M111.320929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acker MG, Kolitz SE, Mitchell SF, Nanda JS, Lorsch JR. Reconstitution of yeast translation initiation. Methods Enzymol. 2007;430:111–145. doi: 10.1016/S0076-6879(07)30006-2. [DOI] [PubMed] [Google Scholar]
- 20.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 21.Carlson BA, Hatfield DL. Transfer RNAs that insert selenocysteine. Methods Enzymol. 2002;347:24–39. doi: 10.1016/s0076-6879(02)47005-x. [DOI] [PubMed] [Google Scholar]
- 22.Kothe U, Paleskava A, Konevega AL, Rodnina MV. Single-step purification of specific tRNAs by hydrophobic tagging. Anal Biochem. 2006;356:148–150. doi: 10.1016/j.ab.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 23.Jan E, Kinzy TG, Sarnow P. Divergent tRNA-like element supports initiation, elongation, and termination of protein biosynthesis. Proc Natl Acad Sci U S A. 2003;100:15410–15415. doi: 10.1073/pnas.2535183100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donovan J, Copeland PR. Selenocysteine Insertion Sequence Binding Protein 2L Is Implicated as a Novel Post-Transcriptional Regulator of Selenoprotein Expression. PLoS One. 2012;7:e35581. doi: 10.1371/journal.pone.0035581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu XM, Mix H, Carlson BA, Grabowski PJ, Gladyshev VN, Berry MJ, Hatfield DL. Evidence for direct roles of two additional factors, SECp43 and soluble liver antigen, in the selenoprotein synthesis machinery. J Biol Chem. 2005;280:41568–41575. doi: 10.1074/jbc.M506696200. [DOI] [PubMed] [Google Scholar]
- 26.Mongelard F, Bouvet P. Nucleolin: a multiFACeTed protein. Trends Cell Biol. 2007;17:80–86. doi: 10.1016/j.tcb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Hirosawa-Takamori M, Ossipov D, Novoselov SV, Turanov AA, Zhang Y, Gladyshev VN, Krol A, Vorbrüggen G, Jäckle H. A novel stem loop control element-dependent UGA read-through system without translational selenocysteine incorporation in Drosophila. FASEB J. 2009;23:107–113. doi: 10.1096/fj.08-116640. [DOI] [PMC free article] [PubMed] [Google Scholar]