Abstract
We have previously reported that mature adipocyte-derived dedifferentiated fat (DFAT) cells have a high proliferative activity and the potential to differentiate into lineages of mesenchymal tissue similar to bone marrow mesenchymal stem cells (MSCs). In the present study, we examined the effects of autologous DFAT cell transplantation on bone regeneration in a rabbit bone defect model and an ovariectomy (OVX)-induced osteoporosis model. The formation of tissue-engineered bone (TEB) was observed when rabbit DFAT cells were loaded onto a β-tricalcium phosphate (TCP)/collagen sponge and cultured in an osteogenic differentiation medium for 3 weeks. Autologous implantation of DFAT cell-mediated TEB constructs promoted bone regeneration in a rabbit tibial defect model. Regenerated bone tissue induced by transplantation of DFAT cell-mediated TEB constructs was histologically well differentiated and exhibited higher bone strength in a three-point bending test compared to that induced by the β-TCP/collagen sponge alone. In OVX-induced osteoporosis model rabbits, DFAT cells were obtained with the osteogenic activity similar to cells from healthy rabbits. Intrabone marrow injection of autologous DFAT cells significantly increased the bone mineral density (BMD) at the injected site in the OVX rabbits. Transplanted DFAT cells remained mainly on the injection side of the bone marrow by at least 28 days after intrabone marrow injection and a part of them expressed osteocalcin. In conclusion, these results demonstrate that autologous implantation of DFAT cells contributed to bone regeneration in a rabbit bone defect model and an OVX-induced osteoporosis model. DFAT cells may be an attractive cell source for cell-based bone tissue engineering to treat nonunion fractures in all patients, including those with osteoporosis.
Introduction
Cell-based therapies and tissue-engineered approaches have become potential therapeutic strategies for bone repair and metabolic bone disease. Having an optimal cell source for generating functional osteoblasts is critical to achieve clinical success with these therapeutic strategies. Mesenchymal stem cells (MSCs) are multipotent somatic stem cells that can differentiate into a variety of cell types such as osteoblasts, chondrocytes, myocytes, and adipocytes.1 MSCs were originally isolated from bone marrow, but they also can be isolated from other connective tissues such as adipose tissue, periosteum, synovium, and deciduous teeth. Recent studies demonstrated that MSCs offer a promising source of cells for tissue engineering of bone tissue. The osteogenic potential of MSCs has already been applied in several clinical situations such as fracture nonunion, osteogenesis imperfecta, posterior spinal fusion, distraction osteogenesis, and osteoarthritis.2 It has been shown that the number of MSCs in tissue and their proliferative activity is reduced according to their donor's age,3 which results in difficulties in preparing MSCs in large-enough amounts for cell therapy in elderly patients. In addition, MSCs isolated from patients with osteoporosis exhibit a low proliferative activity and low ability to differentiate into the osteogenic lineage.4–6 These results suggest the need for an alternative cell source that can be easily isolated and expanded, especially in elderly subjects and in patients with metabolic bone disorders.
The dedifferentiation process has been demonstrated as a physiological property of the amphibian species, such as during limb regeneration.7 In mammals, terminally differentiated cells are normally incapable of reversing the differentiation process. However, using an in vitro dedifferentiation strategy, which is referred to as the ceiling culture method, fully differentiated adipocytes can switch their phenotype to a more primitive one and gain extensive cell proliferative ability.8 Our group has established a preadipocyte cell line derived from mature adipocytes of ddY mice, and designated these cells as dedifferentiated fat (DFAT) cells.9 We have reported that DFAT cells have a high proliferative activity and, similar to bone marrow MSCs, have the potential to differentiate into mesenchymal tissue lineages.10 In response to specific culture conditions, DFAT cells can differentiate into adipocytes, osteoblasts, chondrocytes, myofibroblasts, skeletal myocytes, and cardiomyocytes.10–13 Transplantation of DFAT cells into injured tissue contributes to regeneration of damaged tissues, including the bladder,11 urethra,14 heart,13 and spinal cord.15 Because DFAT cells can be obtained and expanded from small amounts of subcutaneous adipose tissue in donors regardless of their age,10 DFAT cells could potentially be used in cell-based therapies for a variety of diseases, including metabolic bone disorders, such as osteoporosis, which commonly affect elderly subjects. However, it is still unclear whether DFAT cells contribute to bone regeneration in vivo, especially in subjects suffering from osteoporosis. The aim of this study was to examine the effects of autologous DFAT cell transplantation on bone regeneration in a rabbit bone defect model and an ovariectomy (OVX)-induced osteoporosis model. The results demonstrated that the DFAT cell is a useful cell source for tissue engineering-based bone regeneration.
Materials and Methods
Animals and reagents
Male and female Japanese white rabbits (age: ≥6 months; body weight: 2.0–2.5 kg) and male Sprague-Dawley (SD) rats (8 weeks old) were purchased from CLEA Japan (Tokyo, Japan). The rabbits were fed a CR-3 diet (Nippon Formula Feed Manufacturing, Yokohama, Japan) and given free access to drinking water. All animal experiments were performed in the laboratory according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No.85-23, revised 1996) after approval by the Animal Experiment Committee of Nihon University School of Medicine.
Isolation and ceiling culture of DFAT cells
Isolation of mature adipocytes from fat tissue was performed using a modification of a previously described method.8 Briefly, mature adipocytes were obtained by resection of the subcutaneous adipose tissue from the inguinal region of rabbits. Approximately 1 g of fat tissues were minced and digested in a 0.1% (W/V) collagenase solution (Collagenase Type I; Koken Co., Ltd., Tokyo, Japan) at 37°C for 1 h under gentle agitation. After filtration and centrifugation at 135 g for 3 min, the floating top layer containing unilocular adipocytes was collected. After washing with phosphate-buffered saline (PBS), 5×104 cells were placed in T-12.5 culture flasks (NUNC, Rochester, NY) that were completely filled with the Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with 20% fetal bovine serum (FBS; JRH bioscience, Lot 6G2146, Lenexa, KS) and incubated at 37°C, 5% CO2. The cells floated up and adhered to the top inner ceiling surface of the flask. After 7 days, the medium was removed and the flasks were turned upside-down so that the cells were oriented on the bottom. The medium was changed every 4 days until the cells reached confluence. After splitting, the cells were used for experiments before they reached passage 5.
In vitro differentiation assay
For osteogenic differentiation, DFAT cells were plated in 35-mm dishes (BD Falcon, Franklin Lakes, NJ) at 5×104 cells per dish and grown to confluence. Cells were incubated for 3 weeks in the osteogenic medium consisting of DMEM, 10% FBS, 100 nM dexamethasone (Sigma-Aldrich, St. Louis, MO), 10 mM β-glycerophosphate (Sigma-Aldrich), and 0.05 mM L-ascorbic acid-2-phosphate (Sigma-Aldrich). A fresh induction medium was replaced every 3 days. At indicated time periods, differentiated cells were fixed for 1 h with 4% paraformaldehyde and rinsed with PBS. For the detection of the alkaline phosphatase (ALP) activity, cells were incubated at 37°C for 1 h with 0.16% naphthol AS-TR phosphate (Sigma-Aldrich) and 0.8% Fast Blue BB (Wako, Osaka, Japan) dissolved in a 0.1 M Tris buffer (pH 9.0). For the detection of calcium deposition, cells were incubated with 1% Alizarin Red S (Sigma-Aldrich) for 3 min. Soluble osteocalcin levels in cultures were detected using an enzyme-linked immunosorbent assay (ELISA, Takara Bio, Tokyo, Japan). To create tissue-engineered bone (TEB) constructs, β-tricalcium phosphate (TCP)/collagen I sponges (5×5×5 mm, Collagraft®; NeuColl, Campbell, CA) were soaked in DFAT cell suspension (1×106 cells/mL) for 2 h in a CO2 incubator, and transferred into a 24-well plate (BD Falcon) in 2 mL of the osteogenic medium. The medium was renewed twice a week, and culture was maintained for 3 weeks. The constructs were fixed with 4% paraformaldehyde, rinsed with PBS, and stained for ALP activity and mineralization using Alizarin Red S.
Autologous grafting of the TEB constructs in rabbits
DFAT cells were prepared from 8-month-old male rabbits (n=10) and expanded as described above. β-TCP/collagen I sponges (10×5×5 mm) were soaked in DFAT cell suspension (106 cells/mL) for 2 h, followed by incubation with the osteogenic medium for 2 weeks to create TEB constructs. Segmental bone defects (10×5 mm) of the bilateral middle of tibial shafts were created with an oscillating saw in the 10-month-old rabbits under intravenous pentobarbital (20 mg/kg) and general isoflurane (2%) anesthesia. After washing twice with the DMEM, the TEB constructs were transplanted into the left tibial defects. As a control, β-TCP/collagen I sponges alone were transplanted into the right tibial defects. The muscle attachment was repaired and the skin was closed in layers. At 4 and 8 weeks after transplantation, the rabbits were sacrificed, tibias excised, and frozen at −20°C in plastic tubes (each period n=5).
Microcomputer tomography scanning
Before microcomputer tomography (micro-CT) analysis, both frozen tibias were thawed and immersed in a saline solution throughout the analysis. Parameters of mass and architecture were studied using micro-CT histomorphometry with microfocus X-ray CT scanning (Scan Xmate-A090S; Comscantecno Co, Kanagawa, Japan). The system was set to 100 mA and 37 kV, and the voxel size was 30.41 μm. Relative bone volume (bone volume/total volume: BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), and trabecular separation (Tb.Sp) of implants were calculated by using Luzex-F software (Nireco, Tokyo, Japan).
Mechanical loading and bone strength
After micro-CT analysis, both tibias were carefully cleaned of any remaining adherent soft tissue, and subjected to a three-point bending test. The whole tibia was placed on the lower supports of a three-point bending fixture with the posterior side facing downward in the autograph (Shimadzu Co., AG-2000E, Kyoto, Japan). The upper loading device was aligned to the center of the tibial shaft corresponding to the bone defect sites. The load was applied at a constant displacement rate of 2 mm/min until failure. The load and displacement were recorded by the testing machine software (Shimadzu Co.). The locations for maximum load (N) at failure, energy absorbed (N.mm) were selected manually from the load and displacement curve and calculated as described previously.16
Histological analysis
The grafts were harvested at 4 and 8 weeks after transplantation. The specimens were fixed in 70% ethanol and embedded in glycolmethacrylate (Wako Pure Chemical, Osaka, Japan) without decalcification. Samples from the middle of the specimens were cut into 5-μm sections and stained with hematoxylin–eosin (HE), toluidine blue (TB), and Villanueva-Goldner (VG) for examination by light microscopy (Olympus BX50; Olympus, Tokyo, Japan).
The osteoporosis model and DFAT cell transplantation
The postmenopausal osteoporosis model induced by OVX in eight 10-month-old female Japanese white rabbits was performed as described previously.17 Before OVX and 5 months after, bone mineral density (BMD) was measured in the bilateral femurs using dual energy X-ray absorptiometry (QDR 4500A; Hologic, Bedford, MA) to confirm the development of osteoporosis. After confirming reduction of BMD, the subcutaneous fat tissue in each rabbit was extracted and DFAT cells were prepared as described above. The cells at passage 1 were stored in liquid nitrogen until use for transplantation. A portion of the cells was expanded and examined for osteogenic potential to form TEB constructs as described above. At 7 months after OVX, DFAT cells (1×108) were suspended in 2 mL of saline and injected into the right femoral bone marrow using an 18G needle. In the left femoral bone marrow, 2 mL of saline was injected as a control. After injection, infusion holes were closed with cyanoacrylate on both sides. BMD was measured in bilateral femurs by dual energy X-ray absorptiometry at 4 weeks after DFAT injection. The rabbits were then sacrificed and the femurs were excised, fixed in 100% ethanol, and the cancellous BMD was measured in the femoral neck using the micro-CT system (eXplore Locus; GE Healthcare Biosciences, Waukesha, WI). Histological evaluation was performed by VG staining to examine new bone formation in each femur.
Evaluation of engraftment of DFAT cells
Green fluorescent protein (GFP)-labeled DFAT cells derived from a GFP transgenic rat (SD TgN [act-EGFP] Osb-EGFP)13 (1×105) were suspended in 100 μL of saline and injected into the right femoral bone marrow of SD rats using an 18G needle. After injection, infusion holes were closed with cyanoacrylate. At indicated time periods, the rats were sacrificed and the femurs were excised. The samples were fixed in a formalin-based fixative, embedded in 8% gelatin in PBS. Samples were snap-frozen with liquid nitrogen, cut into sections of 5 μm, and subjected to fluorescent microscopy (Nikon Eclipse TE 2000-U; Nikon, Tokyo, Japan). On day 28, bone marrow cells were isolated by flushing the femurs, fixed, and permeabilized using a permeabilization reagent (BD Biosciences). The cells were incubated with the mouse monoclonal anti-osteocalcin antibody (1:100; Abcam, Cambridge, MA) followed by incubation with the allophycocyanin-conjugated anti-mouse IgG antibody (BD Bioscience). Fluorescence intensity was analyzed by using flow cytometry (FACSCalibur; Becton Dickinson, Franklin Lakes, NJ) with FlowJo software (Tree Star, Ashland, OR). 7-AAD was used to exclude dead cells. The cells were also centrifuged onto glass slides using cytospin and were then fixed and stained with the mouse monoclonal anti-osteocalcin antibody (1:200; Abcam) and the rabbit anti-GFP antibody (1:100; Medical and Biological Laboratories, Nagoya, Japan), and the secondary anti-mouse IgG-Alexa Fluor 594 and anti-rabbit IgG-Alexa Fluor 488 antibodies (1:500; Invitrogen). The nuclei were stained with Hoechst 33342. Immunofluorescence images were obtained with a confocal microscope (Olympus FV10i; Olympus) equipped with 60×oil immersion optics.
Cytokine antibody array
Human DFAT cells and adipose-derived stem cells (ASCs) were prepared as described previously.10 Human bone marrow MSCs were purchased from Lonza (Walkersville, MD). DFAT cells, ASCs, and bone marrow MSCs were plated in 60-mm dishes at a density of 5×104 cells, and incubated with 10% FBS in the DMEM for 1 week. The cells were then incubated with 0.5% FBS in the DMEM for 3 days. Conditioned media were taken and analyzed for cytokines using an antibody array (RayBiotech, Norcross, GA). The cytokines were measured in accordance with the manufacturer's instructions. The signal was detected using a chemiluminescence imaging system (LAS-3000; Fujifilm, Tokyo, Japan) and quantified using Multi Gauge software (Fujifilm). The positive control was used to normalize the results from the different membranes being compared.
Statistical analysis
All data are presented as the mean±SD. The Mann–Whitney U test was used for comparisons of parameters between the groups with p<0.05 considered as statistically significant (GraphPad Prism ver 5.0, La Jolla, CA).
Results
Rabbit DFAT cells can differentiate into osteoblasts in vitro
We first prepared DFAT cells from rabbit subcutaneous adipose tissue and examined their osteogenic potential in vitro. As many as 3×106 DFAT cells were generated by passage 2 from 5×104 of mature adipocytes that were isolated from less than 1 g of rabbit subcutaneous adipose tissue. DFAT cells exhibited fibroblast-like morphology (Fig. 1A) and the doubling time was approximately 60 h at passage 2. The cells maintained their proliferative potential even at passage 13. When rabbit DFAT cells were cultured in the osteogenic induction medium, the cells maintained their fibroblast-like morphology and exhibited ALP, a characteristic of osteoblasts, from 2 to 4 weeks of culture (Fig. 1B left panel). Mineralized matrix aggregates were observed after 3 weeks of culture by Alizarin Red staining (Fig. 1B right panel). Protein levels of osteocalcin, an osteoblast-associated matrix protein, were significantly (p<0.05) increased at 2 and 3 weeks of osteogenic differentiation culture compared with before induction (Fig. 1C). When rabbit DFAT cells were loaded into βTCP/collagen I sponges and cultured in the osteogenic medium for 3 weeks, the cells exhibited positive staining for ALP (Fig. 1D, upper panel) and for Alizarin Red (Fig. 1D, lower panel). These results indicate that rabbit DFAT cells possess osteogenic differentiation capability and can form TEB constructs in vitro.
FIG. 1.
Osteogenic differentiation of rabbit dedifferentiated fat (DFAT) cells in vitro. (A) Morphology of rabbit DFAT cells. Scale bar: 200 μm. (B) Confluent DFAT cells were cultured with the osteogenic medium for 3 weeks. Cells were stained for the alkaline phosphatase activity (ALP) and for Alizarin Red S (Alizarin Red) to visualize the calcified extracellular matrix. Scale bar: 200 μm. (C) Protein levels of osteocalcin were measured by enzyme-linked immunosorbent assay (ELISA) in DFAT cells before and after osteogenic differentiation culture. Data are represented as mean±SD (n=3). *p<0.05 versus 0W. (D) DFAT cells were seeded on β-tricalcium phosphate (TCP)/collagen I sponges (Collagraft®) and cultured in the osteogenic medium for 3 weeks. The constructs were stained for ALP and for Alizarin Red. Scale bar: 1 mm.
Autologous implantation of DFAT cells promotes bone regeneration in a rabbit bone defect model
We next examined whether autologous DFAT transplantation contributes to bone regeneration in a rabbit tibial defect model. After 4 and 8 weeks from transplantation, tibial bones were removed and analyzed for trabecular bone microarchitecture by micro-CT. Mature callus with neocortical mineral deposition was frequently observed in DFAT cell-mediated TEB grafts at 8 weeks (Fig. 2A arrowheads). In contrast, almost no neocortex formation was observed in control grafts. Micro-CT parameters revealed that BV/TV and Tb.N values in the DFAT cell-mediated TEB grafts were significantly higher (p<0.05) at 4 and 8 weeks after transplantation compared with those in the control grafts (Fig. 2B). Also, Tb.Sp value in the DFAT cell-mediated TEB grafts were significantly lower (p<0.05) at 4 and 8 weeks after transplantation compared with the control grafts.
FIG. 2.
Effects of autologous DFAT cell transplantation in a rabbit bone defect model. After segmental bone defects were made in the middle of the bilateral tibial shafts, tissue-engineered bone (TEB) constructs created by β-TCP/collagen I sponges with DFAT cells were self-transplanted into the left side of the defect. In contrast, β-TCP/collagen I sponges alone (without cells) were transplanted into the right side of the defects as a control. After 4 and 8 weeks post-transplantation, bilateral tibial bones were removed and analyzed by micro-CT. The mechanical strength of the bones was then measured by the three-point bending test. (A) Representative micro-CT images of tibial bone defects at 8 weeks post-transplantation. Arrowheads indicate neocortical mineral deposition. (B) Quantitative micro-CT analysis of the grafts. BV/TV: bone volume density, Tb.N: trabecular number, Tb.Sp: trabecular spacing. D: DFAT cell-mediated TEB grafts, C: control grafts. Data are represented as mean±SD (n=5). *p<0.05 versus control. (C) Three-point bending test on the midshaft tibia. Bone strength of midshaft tibia was assessed by maximum load and energy absorbed. Data are represented as mean±SD (n=5). *p<0.05, **p<0.01 versus control.
To examine the bone strength of the grafts, biomechanical evaluation by a three-point bending test was performed. The tibial maximum load increased by 75% (p<0.001) and 47% (p<0.05) at 4 weeks and at 8 weeks after transplantation in the DFAT cell-mediated TEB grafts compared with the control grafts (Fig. 2C). The energy absorbed, another bone strength index, increased by 165% (p<0.001) and 80% (p<0.01) at 4 weeks and at 8 weeks, respectively, in comparison to the control grafts (Fig. 2C).
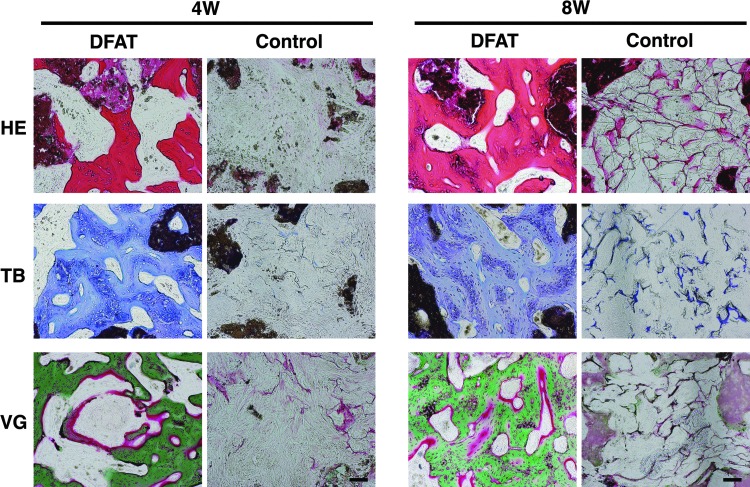
In histological examination at 4 weeks after transplantation, new bone formation was observed in DFAT cell-mediated TEB grafts (Fig. 3). VG staining showed that the newly formed bones consisted largely of mineralized bone tissue (stained in green color) surrounding the unmineralized osteoid surface (in red color). At 8 weeks after transplantation, the mineralized bone tissue filled the spaces within the collagen matrix in DFAT cell-mediated TEB grafts. In contrast, β-TCP/collagen I sponge grafts were almost never replaced by newly formed bone at 4 and 8 weeks after transplantation. These results demonstrate that autologous implantation of DFAT cell-mediated TEB constructs promotes bone regeneration and tissue integration in the rabbit tibial defect model.
FIG. 3.
Representative photomicrographs of the grafts. The grafts were harvested at 4 and 8 weeks post-transplantation. The grafts were sectioned and stained with hematoxylin–eosin (HE), toluidine blue (TB), and Villanueva-Goldner (VG). Scale bar: 100 μm.
Autologous implantation of DFAT cells increases BMD in a rabbit osteoporosis model
We next examined whether autologous DFAT transplantation into bone marrow increases BMD in a rabbit osteoporosis model. At 5 months after OVX, the body weight increased by approximately 200 g compared to before OVX, whereas bilateral femoral BMDs were significantly decreased (p<0.05; −28.4% in left and −27.7% in right) compared to before OVX (Fig. 4B). DFAT cells could be obtained from approximately 1 g of subcutaneous adipose tissue in the OVX-induced osteoporosis rabbits. The DFAT cells derived from OVX rabbits exhibited osteogenic differentiation capacity similar to that of normal rabbits when the cells were cultured in the osteogenic induction medium with the β-TCP/collagen I matrix (Fig. 4A).
FIG. 4.
Effects of autologous DFAT cell transplantation in a rabbit osteoporosis model. DFAT cells were prepared from the rabbits at 5 months after ovariectomy (OVX). The cells (1×108/2 mL saline) were then injected into the right femoral bone marrow at 7 months after OVX. As a control, 2 mL of saline was injected into the left femoral bone marrow at the same time. At 4 weeks after injection, bone analysis was performed. (A) A tissue-engineered bone created from βTCP/collagen I sponges seeded with DFAT cells prepared from the OVX rabbit adipose tissue. Scale bar: 1 mm. (B) Bone mineral density (BMD) in bilateral femurs as measured by dual energy X-ray absorptiometry. Data are represented as mean±SD (n=8). *p<0.05 (Mann–Whitney U teat). (C) Representative micro-CT images of bilateral femoral necks. (D) Cancellous BMD in the bilateral femoral necks. Data are represented as mean±SD (n=8). *p<0.05 (Mann–Whitney U test). (E) Representative VG staining of the bilateral femoral necks. Scale bar: 1 mm. Lt, left; Rt, right.
To investigate the potential for recovery of BMD in DFAT cells, DFAT cells were autologously injected into the right femoral bone marrow in the OVX rabbits. One month later, the femoral BMD showed that there was no apparent change before and after injection on the saline side (control), but that there was a tendency to increase after injection on the DFAT side (Fig. 4B). The femoral BMD after injection was significantly higher (p<0.05) on the DFAT side compared to the saline side. Typical micro-CT images of DFAT- and saline-injected femurs are shown in Figure 4C. Comparison of the longitudinal and transverse cross sections of the bilateral femoral necks at almost the same site showed that the cancellous bone structure was denser on the DFAT side than on the saline side. Micro-CT images of excised femurs showed that the cancellous BMD was significantly higher (p<0.05) on the DFAT side than on the saline side (Fig. 4D). The cancellous bone structure was also denser on the DFAT side in histological preparations (VG staining), thus confirming a difference in new bone formation (Fig. 4E). These findings show that transplantation of DFAT cells into the bone marrow induce an increase in cancellous bone density in the rabbit osteoporosis model.
DFAT cells with osteoblast phenotype were detected in bone marrow after intrabone marrow transplantation
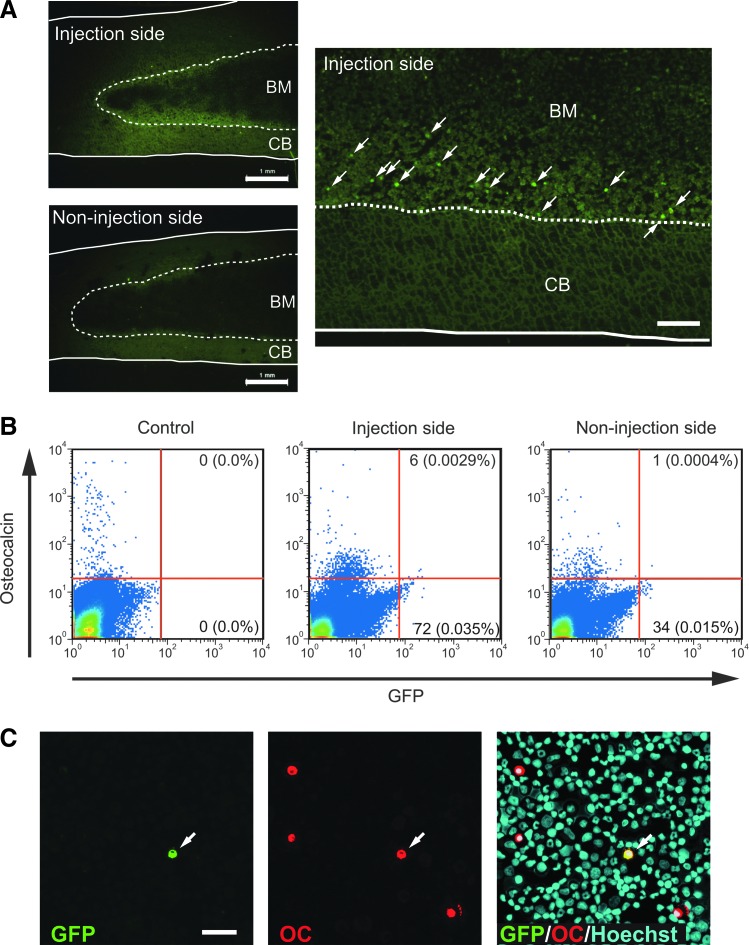
To evaluate engraftment of DFAT cells, GFP-labeled DFAT cells were injected into bone marrow of a femur in rats and the cells were analyzed. Histological analysis of femurs at 7 days after injection revealed that a certain amount of GFP+ cells were observed at the surface of bone trabeculae on the injection side, but not on the noninjection side (Fig. 5A), whereas GFP+ cells could not be detected at 14 and 28 days after injection. To detect GFP+ cells with more sensitive methods, we performed flow cytometry and immunocytochemistry on cytospin preparations of bone marrow cells isolated from femurs. Flow cytometric analysis revealed that GFP+ cells were detected in bone marrow cells from the injection side at 28 days after injection (Fig. 5B). The percentage of GFP+ cells in total bone marrow cells was approximately 0.04%. GFP+ cells were also detected in bone marrow cells from the noninjection side, however, the ratio was lower than was detected on the injection side (0.015%). GFP+ cells expressing osteocalcin were detected in both flow cytometry and cytospin preparations (Fig. 5B, C). Similar results were observed in two independent experiments. These results suggest that, by at least 28 after intrabone marrow transplantation, DFAT cells remained mainly on the injection side of the bone marrow and that a part of them converted to the osteoblast phenotype.
FIG. 5.
Engraftment of DFAT cells in bone marrow after intrabone marrow injection. Green fluorescent protein (GFP)-labeled rat DFAT cells were injected into the left femoral bone marrow of SD rats. (A) On day 7, bilateral femurs were sectioned and subjected to fluorescent microscopy. Arrows indicate GFP+ cells. BM: bone marrow, CB: cortical bone. Scale bar: 1 mm in left panel, 200 μm in right panel. (B) Flow cytometry of bone marrow cells isolated from bilateral femurs at 28 days after injection. Bone marow cells isolated from nontreated rats were used as control. (C) Cytospin preparation of bone marrow cells was immunostained for GFP and osteocalcin (OC). Arrow indicates GFP+ osteocalcin+ cells. Scale bar: 100 μm.
DFAT cells secrete several cytokines associated with bone formation and remodeling
To clarify cytokines associated with bone formation and remodeling in DFAT cells, cytokine array analysis of conditioned media was performed. The data showed that human DFAT cells secreted a variety of cytokines, including interleukin-6 (IL-6), interleukin-8 (IL-8), tissue inhibitors of metalloproteinase-1 and -2 (TIMP-1, -2), insulin-like growth factor binding protein-4 (IGFBP-4), leukemia inhibitory factor (LIF), transforming growth factor-β2, oncostatin M, and osteoprotagenin, which is essential for bone formation and remodeling (Fig. 6A). The expression profiles of DFAT cells were very similar to those of ASCs and bone marrow MSCs (Fig. 6A, C).
FIG. 6.
Protein array analysis of conditioned media. The conditioned media from human DFAT cells (DFAT), adipose-derived stem cells (ASC), and bone marrow mesenchymal stem cells (BM-MSC) were taken and subjected to a protein array analysis. (A) Expression profiles of multiple cytokines. PC: positive control, NC: negative control. (B) Cytokine names corresponding to each of the spots. (C) The intensities of signals were quantified by densitometry.
Discussion
In the present study, we successfully generated TEB constructs from DFAT cells prepared from small amounts of adipose tissue in rabbits. Autologous implantation of DFAT cell-mediated TEB constructs promoted bone regeneration in a rabbit tibial defect model. Although previous studies10,18 demonstrated that human DFAT cells could form the osteoid matrix when implanted subcutaneously into immunodeficient mice, this study is the first to show the therapeutic potential of local DFAT cell transplantation for a segmental bone defect. Regenerated bone tissue induced by DFAT cell transplantation was histologically well differentiated and exhibited high bone strength according to a three-point bending test. Similar bone-forming potential has been reported by using MSCs derived from bone marrow,19,20 adipose tissue,21 and fetal tissue.22 Further investigation is required to assess the bone-forming potential of DFAT cells in comparison with the other types of MSCs. There are likely mechanisms by which MSC-based TEB grafts allow bone regeneration of critical-sized defects. The graft containing osteogenic cells and the highly mineralized extracellular matrix may support the integrity of the defect space, and inhibit the invasion of fibrous tissue, leading to neovascularization in the graft. Subsequently, osteogenic cells may directly and indirectly contribute to bone formation by differentiation into osteoblasts and by recruitment of native osteoblasts from the surrounding tissue through the secretion of certain growth factors and cytokines.2 Similar mechanisms are assumed to occur in the DFAT cell-based TEB because DFAT cells can efficiently differentiate into mineralized osteoblasts in vitro (see Fig. 1) and secrete a variety of cytokines responsible for bone remodeling and angiogenesis (see Fig. 6). IL-6, OSM, and LIF are known as glycoprotein 130-signaling cytokines that bind to osteoblasts, osteoclasts, and chondrocytes in a paracrine manner and regulate the processes of bone formation and bone resorption during normal bone development, growth, and remodeling.23
The present study also demonstrated that DFAT cells could be obtained from adipose tissue in the OVX-induced osteoporosis model rabbits. The obtained cells could differentiate into mineralized osteoblasts in vitro, similarly to cells from healthy rabbits. Additionally, intrabone marrow injection of undifferentiated DFAT cells increased BMD at the site of injection in the OVX rabbits. Similar positive effects of intrabone marrow injection treatment for OVX-induced osteoporosis model animals have been reported by using bone marrow MSCs.24 These studies suggest that DFAT cells derived from osteoporosis patients retain the osteogenic activity and that local transplantation of DFAT cells may have therapeutic potential for the treatment of osteoporosis. In the present study, we did not compare the differences in osteogenic potential of bone marrow MSCs and DFAT cells, because we did not succeed in preparing sufficient amounts of bone marrow MSCs for autologous transplantation in the OVX rabbits. The low proliferative ability of bone marrow MSCs in OVX rabbits has been reported previously.25
Cell trace analysis revealed that transplanted DFAT cells were detected on the injection side of bone marrow by 4 weeks after transplantation and that a part of the cells expressed osteocalcin (Fig. 5B, C). The results suggest that at least a part of DFAT cells engrafted onto injected bone marrow tissue and contributed to local osteogenesis by differentiation into the osteoblast phenotype. Since only a very small number of GFP+ osteocalcin+ cells were detected in the present study, it is unlikely that DFAT cell-derived osteoblasts contributed much to bone regeneration. However, the results were obtained under condition of syngeneic cell transplantation into healthy bone marrow. The actual number of engrafting cells may be larger in condition of autologous cell transplantation into osteoporotic bone marrow in the OVX rabbits. Interestingly, engraftment of DFAT cells could be detected on the noninjection side of bone marrow at 28 days after injection (see Fig. 5B). The findings suggest that systemic administration such as intravenous injection of DFAT cells could contribute to an increase of BMD in patients with osteoporosis. Further studies are needed to test the effect of systemic administration of DFAT cells on bone formation in the osteoporosis animal model. Because increased bone resorption by osteoclasts is thought to be the major cause of OVX-induced osteoporosis, our results imply that DFAT cells not only accelerate cancellous bone formation, but also modulate the function of osteoclasts. Our cytokine array analysis revealed that human DFAT cells secrete osteoprotagenin, which acts as a decoy receptor for the receptor activator of nuclear factor-κB ligand (RANKL) (see Fig. 6), suggesting that DFAT cells are a negative regulator of osteoclastogenesis as previously reported in bone marrow MSCs.26
DFAT cells have several properties that make them well-suited for cell-based bone tissue engineering. First, DFAT cells can be obtained from donors regardless of age. In our previous study using human DFAT cells from a total of 18 donors ranging from 4 to 81 years of age, we successfully prepared DFAT cells from every donor.10 We also found that DFAT cells can be obtained from patients with metabolic bone disorders such as osteoporosis and rheumatoid arthritis. The differentiation potential for osteoblasts was confirmed in all the donors examined. These findings, together with the present study, suggest that DFAT cells can be used for autologous transplantation in patients of various ages, including elderly patients with metabolic bone diseases. Second, DFAT cells can be obtained and expanded from a very small amount of adipose tissue. Because adipocytes generate DFAT cells with approximately 40% efficiency in the ceiling culture, only a small number of adipocytes (in the present study 5×104 cells, which can be obtained from approximately 100 mg of adipose tissue) were sufficient to obtain enough DFAT cells for transplantation within a few passages. This property suggests that in clinical use, DFAT cells can be prepared through a less invasive surgical or liposuction procedure. Third, DFAT cells are highly homogenous. DFAT cells contain almost no other cell types, even at the first passage, because the cells are prepared from isolated mature adipocyte fractions.10 This property may lead to higher safety and reproducible effects for clinical applications. Further investigation is required to elucidate the long-term engraftment and safety of DFAT cell transplantation.
Conclusions
These results demonstrate that mature adipocyte-derived DFAT cells possess osteogenic differentiation potential. Autologous implantation of DFAT cells contributes to bone regeneration in a rabbit bone defect model and an OVX-induced osteoporosis model. DFAT cells may be an attractive source for cell-based bone tissue engineering to treat nonunion fractures in all patients, including those with osteoporosis.
Acknowledgments
We acknowledge the support of this study by financial grants from the Ministry of Education, Science, Sports and Culture of Japan (20590707, 23390190, and 80183648) and the “Strategic Research Base Development” Program for Private Universities subsidized by MEXT (S0801033), by a financial grant from the Japan Science and Technology Agency (08030216), and by a Nihon University Multidisciplinary Research Grant (10-027, 11-017). The authors gratefully thank Yoshiki Taniguchi and Chii Yamamoto for their technical support of our experiments.
Disclosure Statement
No competing financial interests exist.
References
- 1.Aldahmash A. Zaher W. Al-Nbaheen M. Kassem M. Human stromal (mesenchymal) stem cells: basic biology and current clinical use for tissue regeneration. Ann Saudi Med. 2012;32:68. doi: 10.5144/0256-4947.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Z.Y. Teoh S.H. Hui J.H. Fisk N.M. Choolani M. Chan J.K. The potential of human fetal mesenchymal stem cells for off-the-shelf bone tissue engineering application. Biomaterials. 2012;33:2656. doi: 10.1016/j.biomaterials.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 3.D'Ippolito G. Schiller P.C. Ricordi C. Roos B.A. Howard G.A. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez J.P. Montecinos L. Rios S. Reyes P. Martinez J. Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem. 2000;79:557. doi: 10.1002/1097-4644(20001215)79:4<557::aid-jcb40>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez J.P. Rios S. Fernandez M. Santibanez J.F. Differential activation of ERK1,2 MAP kinase signaling pathway in mesenchymal stem cell from control and osteoporotic postmenopausal women. J Cell Biochem. 2004;92:745. doi: 10.1002/jcb.20119. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez J.P. Garat S. Gajardo H. Pino A.M. Seitz G. Abnormal osteogenesis in osteoporotic patients is reflected by altered mesenchymal stem cells dynamics. J Cell Biochem. 1999;75:414. doi: 10.1002/(sici)1097-4644(19991201)75:3<414::aid-jcb7>3.3.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Brockes J.P. Amphibian limb regeneration: rebuilding a complex structure. Science. 1997;276:81. doi: 10.1126/science.276.5309.81. [DOI] [PubMed] [Google Scholar]
- 8.Sugihara H. Yonemitsu N. Miyabara S. Yun K. Primary cultures of unilocular fat cells: characteristics of growth in vitro and changes in differentiation properties. Differentiation. 1986;31:42. doi: 10.1111/j.1432-0436.1986.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 9.Yagi K. Kondo D. Okazaki Y. Kano K. A novel preadipocyte cell line established from mouse adult mature adipocytes. Biochem Biophys Res Commun. 2004;321:967. doi: 10.1016/j.bbrc.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto T. Kano K. Kondo D. Fukuda N. Iribe Y. Tanaka N. Matsubara Y. Sakuma T. Satomi A. Otaki M. Ryu J. Mugishima H. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215:210. doi: 10.1002/jcp.21304. [DOI] [PubMed] [Google Scholar]
- 11.Sakuma T. Matsumoto T. Kano K. Fukuda N. Obinata D. Yamaguchi K. Yoshida T. Takahashi S. Mugishima H. Mature, adipocyte derived, dedifferentiated fat cells can differentiate into smooth muscle-like cells and contribute to bladder tissue regeneration. J Urol. 2009;182:355. doi: 10.1016/j.juro.2009.02.103. [DOI] [PubMed] [Google Scholar]
- 12.Kazama T. Fujie M. Endo T. Kano K. Mature adipocyte-derived dedifferentiated fat cells can transdifferentiate into skeletal myocytes in vitro. Biochem Biophys Res Commun. 2008;377:780. doi: 10.1016/j.bbrc.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 13.Jumabay M. Matsumoto T. Yokoyama S. Kano K. Kusumi Y. Masuko T. Mitsumata M. Saito S. Hirayama A. Mugishima H. Fukuda N. Dedifferentiated fat cells convert to cardiomyocyte phenotype and repair infarcted cardiac tissue in rats. J Mol Cell Cardiol. 2009;47:565. doi: 10.1016/j.yjmcc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Obinata D. Matsumoto T. Ikado Y. Sakuma T. Kano K. Fukuda N. Yamaguchi K. Mugishima H. Takahashi S. Transplantation of mature adipocyte-derived dedifferentiated fat (DFAT) cells improves urethral sphincter contractility in a rat model. Int J Urol. 2011;18:827. doi: 10.1111/j.1442-2042.2011.02865.x. [DOI] [PubMed] [Google Scholar]
- 15.Ohta Y. Takenaga M. Tokura Y. Hamaguchi A. Matsumoto T. Kano K. Mugishima H. Okano H. Igarashi R. Mature adipocyte-derived cells, dedifferentiated fat cells (DFAT), promoted functional recovery from spinal cord injury-induced motor dysfunction in rats. Cell Transplant. 2008;17:877. doi: 10.3727/096368908786576516. [DOI] [PubMed] [Google Scholar]
- 16.Tanizawa T. Yamaguchi A. Uchiyama Y. Miyaura C. Ikeda T. Ejiri S. Nagal Y. Yamato H. Murayama H. Sato M. Nakamura T. Reduction in bone formation and elevated bone resorption in ovariectomized rats with special reference to acute inflammation. Bone. 2000;26:43. doi: 10.1016/s8756-3282(99)00236-7. [DOI] [PubMed] [Google Scholar]
- 17.Cao T. Shirota T. Yamazaki M. Ohno K. Michi K.I. Bone mineral density in mandibles of ovariectomized rabbits. Clin Oral Implants Res. 2001;12:604. doi: 10.1034/j.1600-0501.2001.120608.x. [DOI] [PubMed] [Google Scholar]
- 18.Justesen J. Pedersen S.B. Stenderup K. Kassem M. Subcutaneous adipocytes can differentiate into bone-forming cells in vitro and in vivo. Tissue Eng. 2004;10:381. doi: 10.1089/107632704323061744. [DOI] [PubMed] [Google Scholar]
- 19.Bruder S.P. Kraus K.H. Goldberg V.M. Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998;80:985. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Bruder S.P. Kurth A.A. Shea M. Hayes W.C. Jaiswal N. Kadiyala S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998;16:155. doi: 10.1002/jor.1100160202. [DOI] [PubMed] [Google Scholar]
- 21.Cowan C.M. Shi Y.Y. Aalami O.O. Chou Y.F. Mari C. Thomas R. Quarto N. Contag C.H. Wu B. Longaker M.T. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z.Y. Teoh S.H. Chong M.S. Lee E.S. Tan L.G. Mattar C.N. Fisk N.M. Choolani M. Chan J. Neo-vascularization and bone formation mediated by fetal mesenchymal stem cell tissue-engineered bone grafts in critical-size femoral defects. Biomaterials. 2010;31:608. doi: 10.1016/j.biomaterials.2009.09.078. [DOI] [PubMed] [Google Scholar]
- 23.Sims N.A. Walsh N.C. GP130 cytokines and bone remodelling in health and disease. BMB Rep. 2010;43:513. doi: 10.5483/bmbrep.2010.43.8.513. [DOI] [PubMed] [Google Scholar]
- 24.Ocarino Nde M. Boeloni J.N. Jorgetti V. Gomes D.A. Goes A.M. Serakides R. Intra-bone marrow injection of mesenchymal stem cells improves the femur bone mass of osteoporotic female rats. Connect Tissue Res. 2010;51:426. doi: 10.3109/03008201003597049. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z. Goh J. Das De S. Ge Z. Ouyang H. Chong J.S. Low S.L. Lee E.H. Efficacy of bone marrow-derived stem cells in strengthening osteoporotic bone in a rabbit model. Tissue Eng. 2006;12:1753. doi: 10.1089/ten.2006.12.1753. [DOI] [PubMed] [Google Scholar]
- 26.Bielby R. Jones E. McGonagle D. The role of mesenchymal stem cells in maintenance and repair of bone. Injury. 2007;38(Suppl 1):S26. doi: 10.1016/j.injury.2007.02.007. [DOI] [PubMed] [Google Scholar]