FIG. 3.
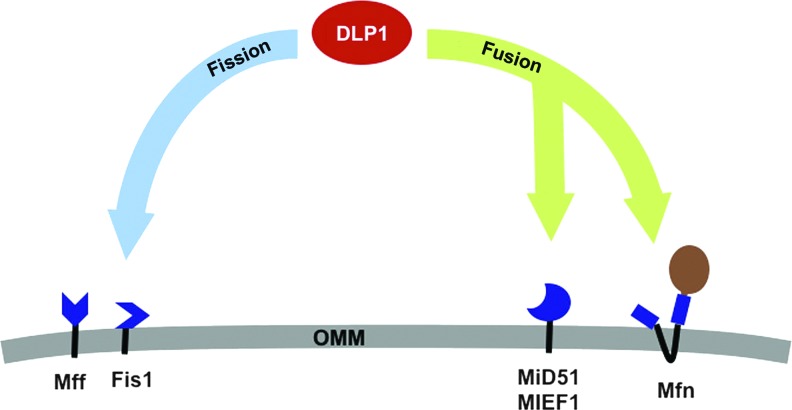
A role of DLP1 interactions controlling the balance of mitochondrial fission and fusion through alternative complex formation. The identification of multiple new receptors at the mitochondrial outer membrane has suggested that enhanced recruitment of DLP1 to the OMM may not necessarily result in enhanced fission. Localized to the OMM, both hFis1 and Mff are profission receptors for DLP1. DLP1 interaction with hFis1 in mammalian systems is associated with enhanced mitochondrial fission. Recently, a second OMM receptor Mff was reported to serve a similar role in the absence of hFis1 protein. Reports differed slightly as to the ability of their overexpression to induce mitochondrial fragmentation and in their requirement to promote apoptosis. Analysis of complexes containing DLP1 at the mitochondria determined the two profission receptors to exist in distinct complexes, suggesting that their functional promotion of fission may occur through differing pathways. MIEF1/MiD51 promotes mitochondrial fusion when overexpressed. Enhanced MIEF1/MiD51 also increases DLP1 recruitment to the OMM while still promoting fusion. DLP1 interaction with MIEF1 alters its GTPase activity, possibly defining an underlying cause. The recent report that DLP1 interaction with Mfn2 supports mitochondrial fusion could also underlie this phenotype. Taken together, recruitment of DLP1 to MIEF1/MiD51 would result in its reduced GTPase activity, still allowing its interaction with Mfn and promoting fusion. While Mff, hFis1, MIEF1/MiD51, and Mfn are bound to the OMM, DLP1 retains the ability to traffic between the cytoplasm and the OMM. DLP1 recruitment to differing specific microdomains of these proteins then represents the regulatable process, likely coordinated by the extensive post-translational modification of DLP1, in directing fission or fusion at the OMM. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars