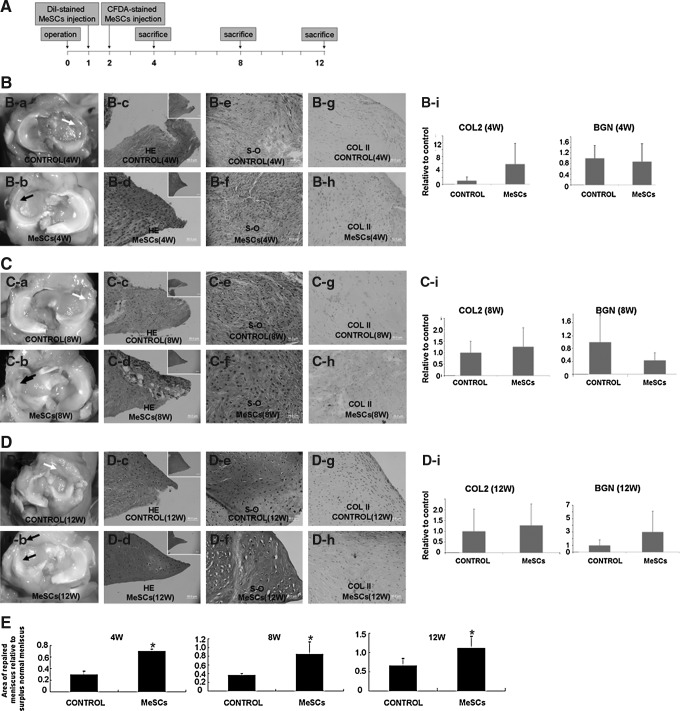
FIG. 2.
Regenerated meniscus at 4, 8, and 12 weeks postmeniscectomy. Experimental design for utilizing MeSCs for in vivo treatment of meniscus injury (A). Gross morphology and typical HE staining of control group (white arrow) and MeSC-treated group (B-a, B-d, C-a, C-d, D-a, D-d; black arrow) at 4, 8, and 12 weeks postmeniscectomy. Real-time PCR analysis of gene expression of COL2 and BGN (B-i, C-i, D-i) in vivo at 4, 8, and 12 weeks postmeniscectomy. Safranin O staining and immunohistochemical staining of control group and MeSC-treated group (B-e, B-f, C-e, C-f, D-e, D-f) at 4, 8, and 12 weeks postmeniscectomy. (E) The sequential area ratio of the repaired meniscus relative to surplus normal meniscus (n=3 for 4 and 8 weeks, n=8 for 12 weeks each group). Scale bars=50 μm (B, C, D), 200 μm (inset of B, C, D). HE, hematoxylin and eosin staining; PCR, polymerase chain reaction; COL2, collagen type II; BGN, biglycan; S-O, safranin O staining; W, weeks.