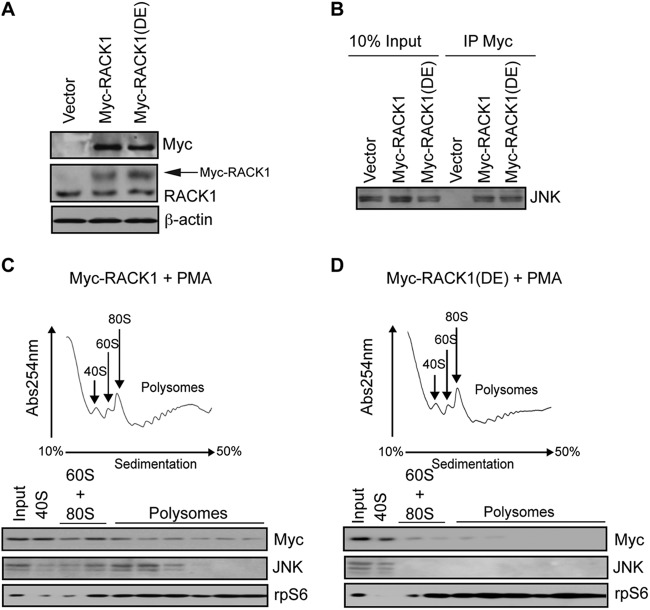
Fig 3.
RACK1 recruits JNK to the ribosome. (A) 30 μg of the corresponding cell lysates was loaded onto SDS-PAGE gels, and the expression of endogenous RACK1 and exogenous Myc-RACK1 and Myc-RACK1R38D/K40E [Myc-RACK1(DE)] mutant in HEK293T cells was monitored by Western blotting with the indicated antibodies. Exogenous Myc-RACK1 variants are indicated by an arrow. β-Actin served as a loading control. (B) Portions (1 μg) of total cell extracts from the cells described in panel A were immunoprecipitated with an anti-Myc antibody (IP Myc), and the amount of JNK associated with Myc-RACK1 and Myc-RACK1R38D/K40E was determined by Western blotting. Inputs (10%) are shown in the left panel. (C and D) Polysome profiles of HEK23T cells transfected with Myc-RACK1 (C) or Myc-RACK1R38D/K40E (D) and treated with 50 nM PMA for 30 min. Then, 10 OD260 units of the corresponding cytoplasmic extracts were sedimented by centrifugation on 10 to 50% sucrose gradients, and the distribution of the indicated proteins across the gradient was monitored by Western blotting. rpS6 was used as a loading control.