Fig 6.
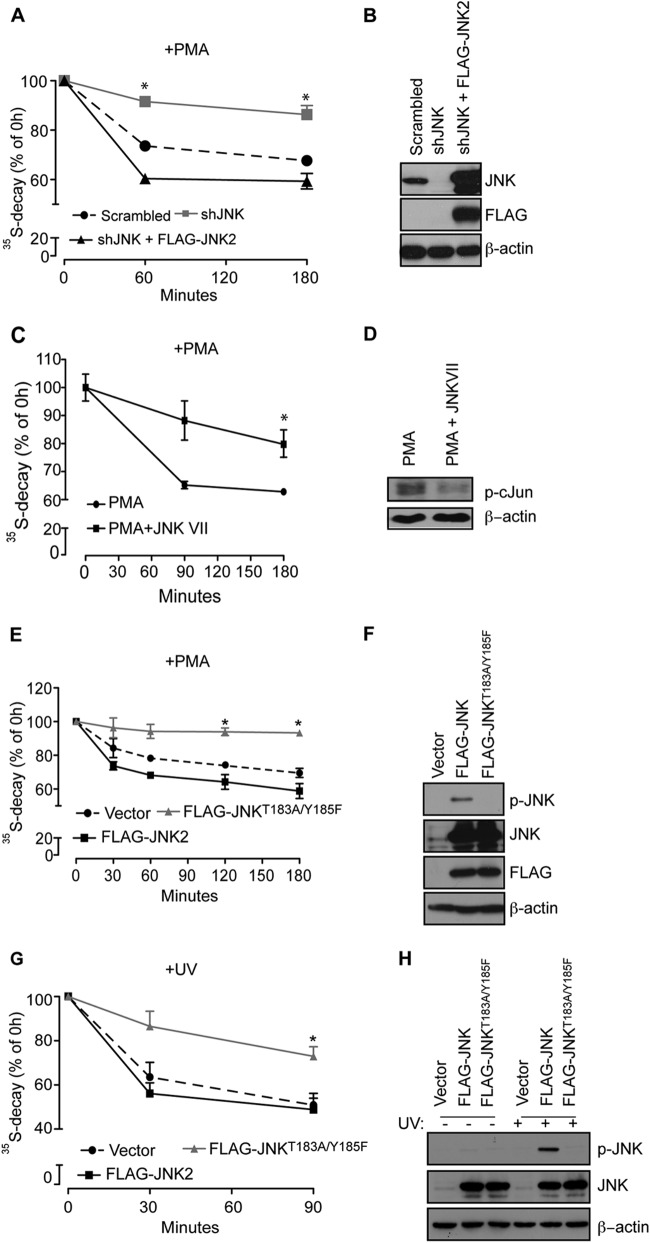
Kinase activity of JNK is required for degradation of NSPs. (A) The stability of NSPs in PMA-treated HEK293T cells was monitored by a [35S]Met-Cys pulse-chase at the 1- and 3-h time points. Values obtained upon transfer of cells to cold media (0 h time point) were set to 100%, and the data are represented as means ± the SEM (n = 3). Depletion of JNK (shJNK) significantly increased stability of 35S-labeled polypeptides compared to Scrambled control and cells in which expression of JNK was rescued with WT JNK2 (shJNK + FLAG-JNK2; P = 0.0101 compared to the Scrambled control; ANOVA, F2,7 = 20.77, P = 0.0038). (B) Portions (30 μg) of the indicated cell lysates were loaded onto SDS-PAGE gels, and the expression of exogenous WT JNK2 (FLAG-JNK2) and endogenous JNK was monitored by Western blotting with the indicated antibodies. β-Actin served as a loading control. (C) HEK293T cells were treated for 3 h with 50 nM PMA alone or in combination with 10 μM JNK-Inhibitor VII (TAT-TI-JIP153-163; JNKVII), and the degradation of NSPs was monitored after the 90-min and 3-h time points. The degradation of NSPs was significantly suppressed by JNK-Inhibitor VII (PMA plus JNK VII) compared to the control (PMA) (Student t test: P = 0.0063 [at 90 min] and P = 0.05 [at 180 min]). Values are represented as means ± the SEM (n = 3). (D) JNK-Inhibitor VII suppressed JNK activity, as monitored by the phosphorylation of c-Jun on Ser63 by Western blotting. Portions (40 μg) of the indicated cell lysates were loaded onto SDS-PAGE gels. β-Actin served as a loading control. (E and H) Degradation of NSPs was monitored by a [35S]Met-Cys pulse-chase in HEK293T cells transfected with FLAG-JNK2 and FLAG-JNK2T183A/Y185F and treated with 50 nM PMA for the indicated time points (E) or exposed to UV light for 90 s (see Materials and Methods) (G). Cells expressing FLAG-JNK2T183A/Y185F exhibited a significant increase in the stability of 35S-labeled polypeptides under both conditions compared to cells expressing FLAG-JNK2 (+PMA [E], P = 0.0114 for FLAG-JNK2T183A/Y185F compared to FLAG-JNK2 [180 min], ANOVA, F2,3 = 60.98, P = 0.0037; +UV [G], P = 0.0086, FLAG-JNK2T183A/Y185F compared to FLAG-JNK2 [90 min], ANOVA, F2,15 = 9.35, P = 0.0023). (F and H) Portions (30 μg) of the indicated cell lysates were loaded onto SDS-PAGE gels, and the levels of FLAG-JNK2 and FLAG-JNK2T183A/Y185F and the phosphorylation of JNK in PMA (F)- or UV (I)-treated cells were monitored by Western blotting. β-Actin served as a loading control.