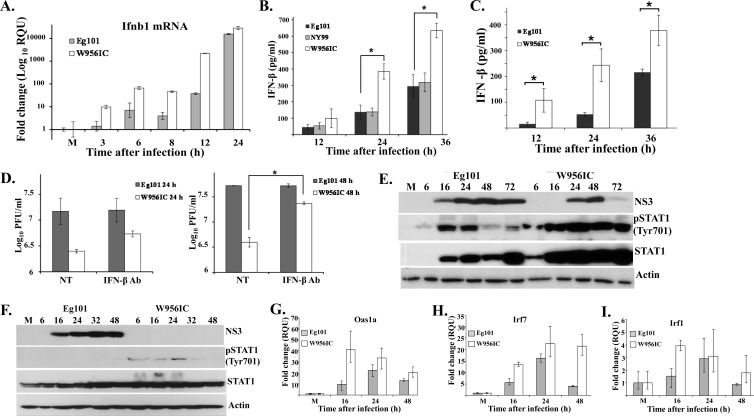
Fig 2.
Analysis of induction of IFN-β by WNV Eg101 and W956IC infections. (A) C3H/He MEFs were mock infected or infected with WNV Eg101 or W956IC at an MOI of 1. Replicate cultures were processed to extract total cell RNA or protein at the indicated times after infection. Ifnb1 mRNA levels detected by real-time qRT-PCR were normalized to the levels of GAPDH in the same sample and are shown as the fold change compared to the level of the Ifnb1 mRNA in mock-infected cells. Samples from each qRT-PCR experiment were assayed in triplicate. Error bars represent the standard errors (SE) of the means. Representative data from one of three independent experiments are shown. M, mock infection. The levels of IFN-β protein in culture fluids harvested from WNV-infected C3H/He (B) or C57BL/6 (C) MEFs were determined by ELISA. Data points are averages from two or more independent experiments performed in duplicate. Bars represent ± standard deviations. Asterisks indicate statistically significant differences (*, P < 0.05). (D) C3H/He MEFs were infected with WNV Eg101 or W956IC at an MOI of 5 for 1 h, and then the cultures were incubated with or without neutralizing antibody (Ab) (1,000 neutralizing units/ml) (PBL Interferon Source) against IFN-β for the duration of the infection. Virus titers in culture fluids were determined at 24 and 48 h after infection by plaque assay on BHK cells. The values shown are averages of duplicate titrations from two experiments. Bars represent ± standard deviations. NT, not treated. NS3 and phosphorylated STAT1 (pSTAT1) or total STAT1 in C3H/He (E) or A549 (F) cell lysates were detected by Western blotting with specific antibodies. Actin was used as the loading control. The blots shown are representative of results obtained from three independent experiments. Changes in mRNA expression levels for the ISGs Oas1a (G), Irf7 (H), and Irf1 (I) in infected C3H/He-infected MEFs were assessed by real-time qRT-PCR as described in panel A.