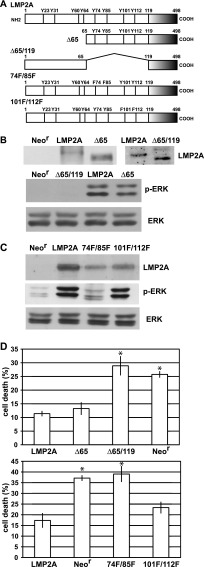
Fig 6.
ITAM is required for LMP2A-mediated induction of anoikis resistance. (A) The LMP2A deletion and point mutants used in this study are illustrated. For the Δ65 mutant, 64 N-terminal amino acids were deleted, and for the Δ65/119 mutant, amino acids 66 to 118 were deleted. The 74F/85F and 101F/112F mutants had double mutations of tyrosine (Y) to phenylalanine (F). The Δ65/119 and 74F/85F mutants had the ITAM mutation. (B) (Top) Mutant LMP2A expression. Cells stably expressing GFP-tagged mutated LMP2A (Δ65 or Δ65/119 mutant cells) were lysed and subjected to immunoprecipitation, followed by Western blotting. Mutated LMP2As were detected by anti-GFP antibody. (Bottom) Results of ERK activation analysis. Neor cells and LMP2A (WT or mutant)-expressing cells were lysed and analyzed by Western blotting. (C) Effect of LMP2A double tyrosine mutation on ERK activation. Cells stably expressing double tyrosine-mutated LMP2A (74F/85F or 101F/112F mutant cells) were lysed and subjected to Western analysis for LMP2A expression (top) and ERK activation (middle and bottom). (D) Analysis of anoikis resistance. Cells with the Δ65 and Δ65/119 mutations (top) and cells with the 74F/85F and 101F/112F mutations (bottom) were cultured in suspension for 48 h, and cell death was analyzed. The representative results of 3 separate experiments using 2 independent clones are represented as the mean percent ± SD. Statistically significant differences were evaluated by Student's t test (*, P < 0.05).