Fig 3.
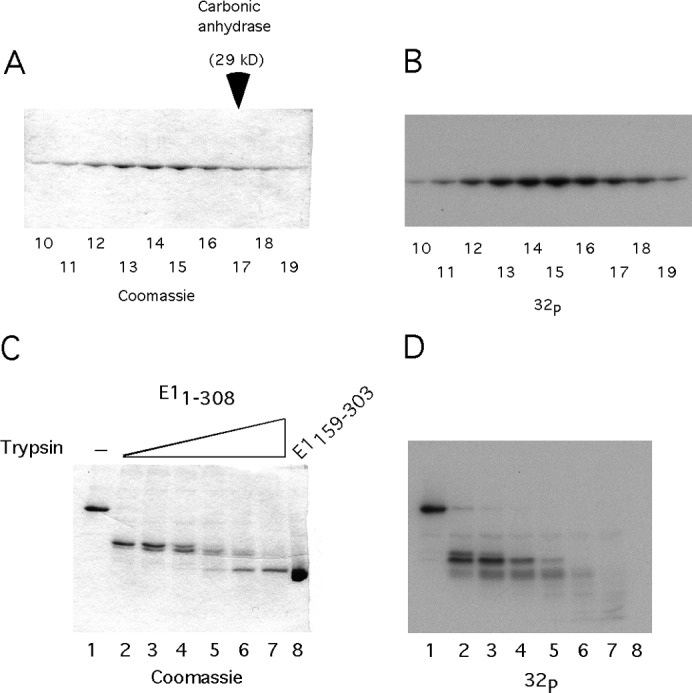
The mechanism of DBD inactivation. (A) A total of 50 ng of E11–308 was phosphorylated in vitro with CK2 and γ32P ATP. The labeled protein was mixed with a 1,000-fold excess of unlabeled E11–308 in the presence of EDTA and analyzed by sedimentation on a 15 to 30% glycerol gradient. The fractions were analyzed by SDS-PAGE, followed by staining with Coomassie and autoradiography. E11–308 peaked in fractions 13 to 15, sedimenting slightly slower than the carbonic anhydrase marker, which peaked in fraction 17. (B) The gel shown in Fig. 3A was dried and subjected to autoradiography. (C) In vitro phosphorylated E11–308 was subjected to partial proteolysis, using 2-fold dilutions of trypsin, followed by SDS-PAGE analysis and staining with Coomassie. (D) The stained gel shown in Fig. 3C was dried and subjected to autoradiography.
