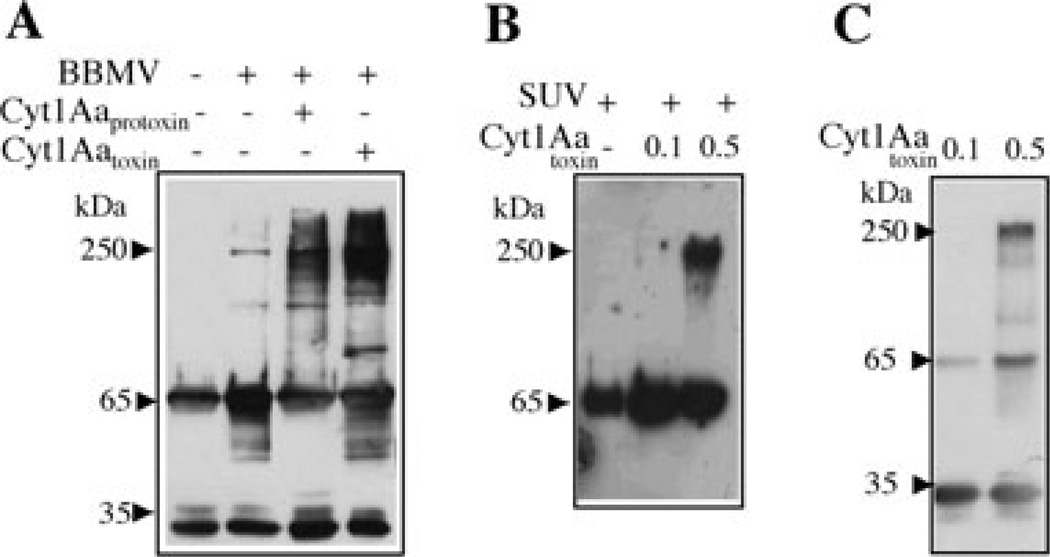
Fig. 1.
Analysis of Cry11Aa oligomer formation by Western blot using polyclonal anti-Cry11A antibody.
A. Activation of soluble Cry11Aa protoxin with trypsin in BBMV isolated from A. aegypti. Incubation was performed for 1 h at 37°C in absence or in the presence of Cyt1Aa-solubilized protoxin or Cyt1Aa-activated toxin. The membrane fraction was separated by centrifugation, suspended in 20 µl of 10 mM CHES, 150 mM KCl, pH 9, loaded in SDS-PAGE and visualized by Western blot. Control without BBMV was also included; this sample was not centrifuged.
B. Activation of soluble Cry11Aa protoxin with trypsin for 1 h at 37°C in SUV (PC : Ch : S mixture) in the presence or absence of different concentrations of Cyt1Aa activated toxin. The membrane fraction was separated by centrifugation as above and Cry11Aa protein was visualized by Western blot.
C. Activation of soluble Cry11Aa protoxin with trypsin in solution for 1 h at 37°C in the presence of different concentrations of Cyt1Aa-activated toxin. Oligomerization of Cry11A was analysed by Western blot. Size of proteins was estimated from molecular pre-stained plus standard, all blue (Bio-Rad).