Figure 4. Strengthening of excitatory transmission and synaptic plasticity in murine brain by engrafting of human glial cells.
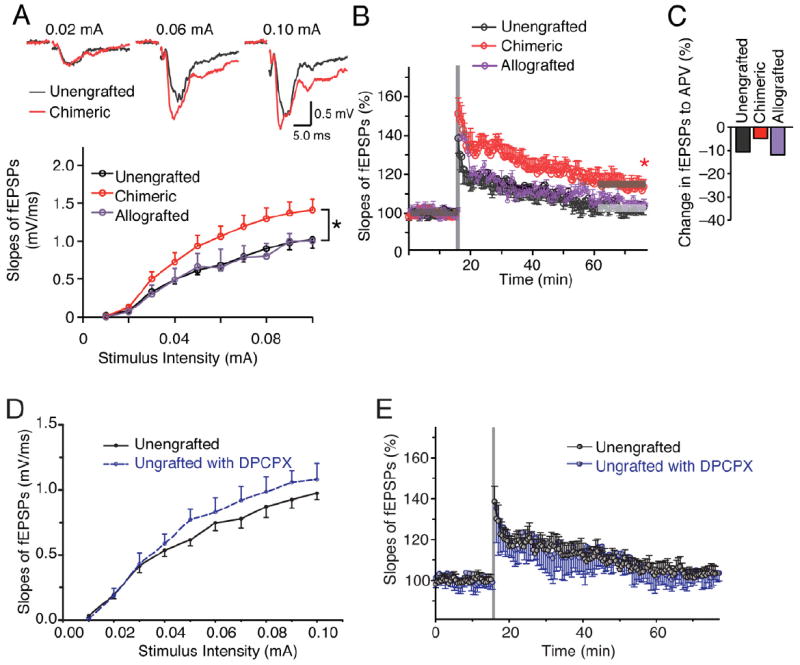
(A) Comparison of field EPSPs (fEPSPs) in humanized chimeric mice and their unengrafted littermate and mouse GPC allografted controls. The slopes of fEPSP were significantly increased in human chimeric mice. (n=3-40; F=3.15, by two-way ANOVA with Bonferroni post hoc t test; *p<0.05) (B) Induction of LTP by 2-trains of high frequency stimulation (each train consisted of 100 pulses at 100 Hz, 30 s between bursts) in human chimeric mice, but not in unengrafted littermates and allografted mice. (n=7 mice each group); *, p < 0.05, t test compared between before and 60 min after the stimulation for each group (C) Relative decreased percentage of fEPSP by addition of NMDA receptor antagonist APV (50μM) in each group (n=15-27). (D) The adenosine A1 receptor antagonist, DPCPX failed to increase the fEPSP slope in unengrafted rag2 controls (100 nM DPCPX, n=8, p>0.05, Bonferroni test). (E) The adenosine A1 receptor antagonist, DPCPX did not decrease the threshold for induction of LTP in unengrafted controls; the fEPSP slope returned to 101.9 ± 3.6% by 60 min after HFS, similar to the rate of extinction in untreated slices (n = 8, t test).
Data graphed as means ± SEM.
See also: Supplementary Figure S3.
