Figure 5. Astrocytic TNFα contributes to LTP facilitation in chimeric mice, which is attenuated by thalidomide.
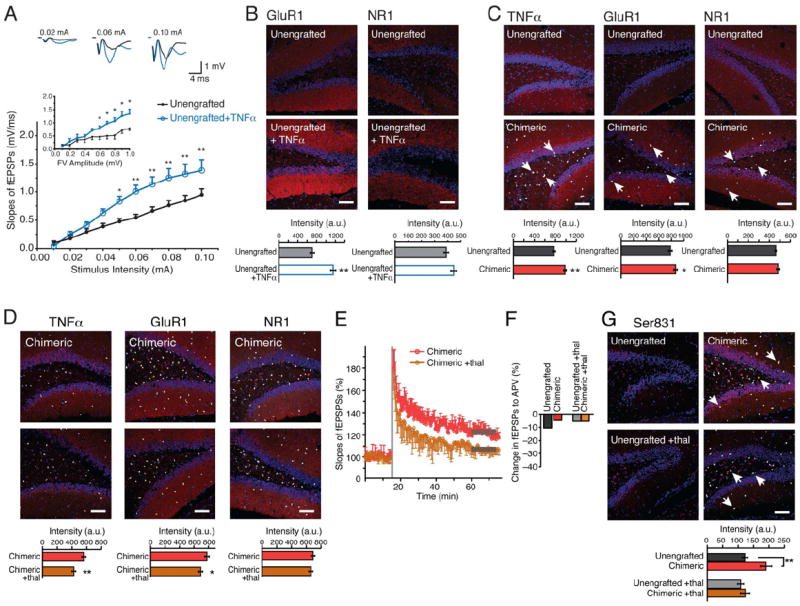
(A) Hippocampal slices prepared from littermate control rag1 mice exhibit a potentiation of fEPSP in response to TNFα (n=6, 12-16 months, *p<0.05; **: p<0.01; Bonferroni post hoc t test). Inset: fEPSP slopes plotted as a function of fiber volley amplitude. (B) Hippocampal slices exposed to TNFα (600 nM; 2-4 hrs.) exhibited an increase in the intensity of GluR1 subunit of AMPA receptors immunolabeling, but not of the NR1 subunit of NMDA receptors (n=5, 9-11months, **p<0.01, t-test). (C) Human chimeric mice exhibit higher intensity of immunolabeling against TNFα and GluR1, but not of NR1 (n=7, 7-20 months, *p<0.05, **p<0.01, t-test). (D) Thalidomide also decreased the immunolabeling of TNFα and GluR1, but not of NR1 in chimeric mice (n=6, 12-16 months, *; p<0.05, **; p<0.01, t-test). (E) The facilitation of LTP in chimeric mice was impaired by thalidomide (n=6, 12.6 ± 0.3 vs. 12.5 ± 0.5 months-old respectively, means ± SEM; p<0.05, t test). (F) Thalidomide did not change the contribution of NMDA receptor activation to fEPSP. Recordings of fEPSPs were obtained before and after addition of the NMDA receptor antagonist APV (50μM), and the difference calculated (n=4). (G) Phosphorylation of the Ser831 site of GluR1 was increased in chimeric mice compared with unengrafted littermate controls. Thalidomide attenuated the increase in phosphorylation of the Ser831 site of GluR1, but had no effect in unengrafted littermate controls, white arrows shows hNuclei+ cells. (n=6, 9-16 months, *p<0.05, t-test).
Scale bar: 100 μm (B,C,D,G). All data graphed as means ± SEM.
See also: Supplementary Figure S4.
