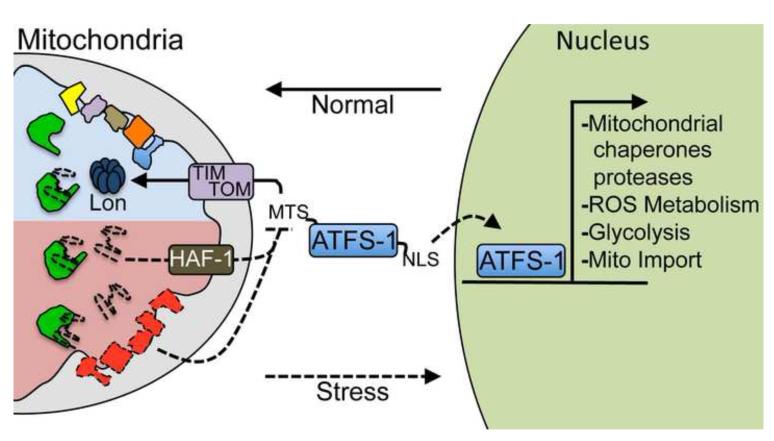
Figure 1. The mitochondrial unfolded protein response.
An illustration of the signaling mechanism that regulates the induction of the mitochondrial unfolded protein response (UPRmt) as elucidated in C. elegans. The UPRmt is activated during mitochondrial dysfunction or stress resulting in the transcriptional up-regulation of protective genes including mitochondrial chaperones and proteases, those involved in reactive oxygen species (ROS) detoxification, the glycolysis pathway and the mitochondrial protein import machinery. The cell determines mitochondrial function and when it is appropriate to induce the UPRmt by monitoring the mitochondrial protein import efficiency of the transcription factor ATFS-1. In the absence of mitochondrial stress, ATFS-1 is translated and efficiently imported into mitochondria via a mitochondrial targeting sequence (MTS), where ATFS-1 is degraded by the Lon protease. However, during mitochondrial dysfunction, general protein import efficiency is reduced allowing a percentage of ATFS-1 to accumulate in the cytosol. Because ATFS-1 also has a nuclear localization sequence (NLS), it then traffics to the nucleus where it induces the UPRmt. Mitochondrial import efficiency can be impaired or reduced by a number of conditions including mitochondrial chaperone depletion or respiratory chain dysfunction. Additionally, import can be slowed by peptide efflux via the ABC transporter HAF-1, which occurs when the mitochondrial chaperone (green) capacity is exceeded by unfolded proteins (dashed lines); all of which result in UPRmt induction to maintain organelle homeostasis.