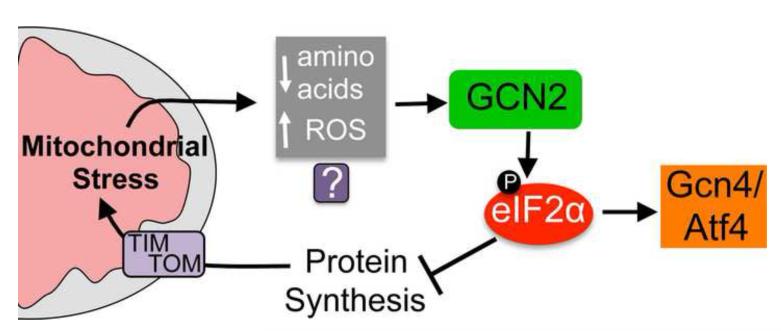
Figure 2. The mitochondrial stress-stimulated translation attenuation pathway.
A model demonstrating how protein synthesis rates are mediated during mitochondrial stress. Conditions including inhibition of a mitochondrial protease required for respiratory chain quality control and mitochondrial ribosome biogenesis [49, 55], respiratory chain or ATP synthase impairment [48] as well as paraquat treatment [55] result in phosphorylation of the translation initiation factor eIF2α by the kinase GCN2. The subsequent decrease in protein synthesis potentially reduces the burden on the protein folding and respiratory chain complex assembly machinery, thus protecting the protein-folding environment. In addition to reducing global protein synthesis, phosphorylated eIF2α promotes the translation and thus activation of the transcription factor Gcn4p in yeast and Atf4 in mammals, both of which are also protective during mitochondrial stress [48, 49, 74]. It is currently unclear how GCN2 becomes activated or senses mitochondrial dysfunction. But, because GCN2 is known to respond to amino acid deprivation and reactive oxygen species (ROS) and both occur during mitochondrial dysfunction multiple possibilities exist.