High-intensity blue light induces increases in [Ca2+]cyt, which are mostly attributed to the function of phot2 and phot1, required for phototropism in Arabidopsis hypocotyls.
Abstract
Phototropins (phot1 and phot2), the blue light receptors in plants, regulate hypocotyl phototropism in a fluence-dependent manner. Especially under high fluence rates of blue light (HBL), the redundant function mediated by both phot1 and phot2 drastically restricts the understanding of the roles of phot2. Here, systematic analysis of phototropin-related mutants and overexpression transgenic lines revealed that HBL specifically induced a transient increase in cytosolic Ca2+ concentration ([Ca2+]cyt) in Arabidopsis (Arabidopsis thaliana) hypocotyls and that the increase in [Ca2+]cyt was primarily attributed to phot2. Pharmacological and genetic experiments illustrated that HBL-induced Ca2+ increases were modulated differently by phot1 and phot2. Phot2 mediated the HBL-induced increase in [Ca2+]cyt mainly by an inner store-dependent Ca2+-release pathway, not by activating plasma membrane Ca2+ channels. Further analysis showed that the increase in [Ca2+]cyt was possibly responsible for HBL-induced hypocotyl phototropism. An inhibitor of auxin efflux carrier exhibited significant inhibitions of both phototropism and increases in [Ca2+]cyt, which indicates that polar auxin transport is possibly involved in HBL-induced responses. Moreover, PHYTOCHROME KINASE SUBSTRATE1 (PKS1), the phototropin-related signaling element identified, interacted physically with phototropins, auxin efflux carrier PIN-FORMED1 and calcium-binding protein CALMODULIN4, in vitro and in vivo, respectively, and HBL-induced phototropism was impaired in pks multiple mutants, indicating the role of the PKS family in HBL-induced phototropism. Together, these results provide new insights into the functions of phototropins and highlight a potential integration point through which Ca2+ signaling-related HBL modulates hypocotyl phototropic responses.
Blue light (BL) is a key factor controlling plant growth and morphogenesis. Recent genetics investigations using Arabidopsis (Arabidopsis thaliana) have revealed that the BL receptors phototropin1 (phot1) and phot2 mediate BL-induced plant movements such as phototropism, chloroplast relocation, stomatal opening, leaf flattening, and leaf positioning responses (Inoue et al., 2010). Most of these responses are mediated redundantly by both phot1 and phot2 (Kinoshita et al., 2001; Sakamoto and Briggs, 2002), but some responses are mediated by either phot1 or phot2 (Sakai et al., 2001; Suetsugu et al., 2005). In addition, several lines of evidence have indicated that phot2 might negatively regulate the phot1-mediated response (de Carbonnel et al., 2010) and vice versa (Harada et al., 2003, 2013).
One of the numerous physiological processes controlled by BL is phototropism. Phototropism enables plants to bend toward incident light by perceiving the direction, wavelength, and intensity of incident light so that they are able to obtain optimum light. Genetic evidence has shown that both phot1 and phot2 redundantly function to regulate hypocotyl phototropism in a fluence-dependent manner (Sakai et al., 2001). Phot1 functions at both low (0.01–1 μmol m−2 s−1) and high (greater than 1 μmol m−2 s−1) fluence rates to mediate phototropic responses, but phot2 functions only at high fluence rates (Inada et al., 2004). The functional specification of phot1 and phot2 could be attributed to the differences in signal intermediates between phot1 and phot2 signaling pathways.
Genetic analysis has illustrated that phot1 mediates hypocotyl phototropism via its downstream signal transducers NONPHOTOTROPIC HYPOCOTYL3 (NPH3; Motchoulski and Liscum, 1999), ROOT PHOTOTROPISM2 (RPT2; Sakai et al., 2000), and NONPHOTOTROPIC HYPOCOTYL4/AUXIN RESPONSE FACTOR7 (NPH4/ARF7; Harper et al., 2000), resulting in the asymmetric distribution of auxin and the induction of a phototropic response in higher plants. Recently, studies have demonstrated that PHYTOCHROME KINASE SUBSTRATE (PKS) proteins are required for hypocotyl phototropism and that PKS1 binds PHOT1 and NPH3 in vivo (Lariguet et al., 2006). In addition, ATP-BINDING CASSETTE B19 (ABCB19), a newly identified auxin transporter, has been reported to interact with phot1 to regulate the BL-dependent phototropism (Christie et al., 2011). However, little is known about phot2-mediated phototropism for functional specialization, especially under high fluence rates of blue light (HBL), although several lines of evidence have shown that phot2- and phot1-mediated signaling pathways share some intermediates in BL responses (Kimura and Kagawa, 2006; Christie, 2007). Previous researches have suggested that phot1 acts not only positively in the presence of RPT2 but also negatively in its absence during the phototropic response of hypocotyls at high fluence rates, suggesting that RPT2 modulates the function of phot1. However, RPT2 does not act in the phot2-mediated pathway (Inada et al., 2004). More recently, RCN1-1, the A1 subunit of Ser/Thr PROTEIN PHOSPHATASE2A (PP2A), has been identified to interact with phot2. While reduced PP2A activity enhances the activity of phot2, it does not enhance either phot1 dephosphorylation or the activity of phot1 in mediating phototropism (Tseng and Briggs, 2010).
Besides these signal intermediates noted above, phototropins may also confer their effects through the change of ion homeostasis. Ca2+ is a case in point. Recent reports have demonstrated that phototropins mediate the mobilization of Ca2+ in response to BL and that phot1 and phot2 mediate Ca2+ increases with distinctive mechanism in leaf cells according to the changes of ambient light intensity (Harada and Shimazaki, 2007). Under low fluence rates of BL, phot1 solely mediated Ca2+ influx through the channels in the plasma membrane. Under HBL, the increase in cytosolic Ca2+ concentration ([Ca2+]cyt) is primarily attributed to phot2-dependent Ca2+ release from the internal calcium stores as well as the plasma membrane Ca2+ channels. Interestingly, the inhibitory effects of phospholipase C (PLC) inhibitors on the BL-induced responses in the wild type are larger than those in the phot1 single mutant, which indicates that there are some functional interactions between phot1 and phot2 to induce the elevation of cytosolic Ca2+ (Harada et al., 2003).
However, until now, the function of Ca2+ in the phototropin-mediated phototropism signaling process has remained largely unknown. Pharmacological experiments indicate that changes in [Ca2+]cyt are required for the phot1-mediated inhibition of hypocotyl growth but not for phot1-mediated phototropism (Folta et al., 2003). Otherwise, electrophysiological studies indicate that phototropic bending involves changes in ion fluxes, including calcium (Babourina et al., 2004). Such divergent responses show that the link between phototropins and calcium has not been firmly established in the case of hypocotyl phototropism. In phototropism, the phot1-dependent relocalization of the auxin efflux carrier PIN-FORMED1 (PIN1) is required for auxin redistribution (Blakeslee et al., 2004), and the PINOID kinase influences the relocalization of PIN1 (Friml et al., 2004). Given that both the calmodulin-related protein TCH3 and the calcium-binding protein AtPBP1 can bind to the PINOID kinase (Benjamins et al., 2003), it would appear that the cross talk among phototropins, auxin, and calcium is an important event for phototropism.
Here, we show that HBL induces increases in [Ca2+]cyt, which are mostly attributed to the function of phot2, and that the increases in [Ca2+]cyt are required for HBL-induced phototropism in Arabidopsis hypocotyls. We also demonstrate that PKS1 may integrate phototropins with auxin transport in phot2-dependent Ca2+ signaling, and we discuss the possible molecular link between phototropins and other potential signal elements in HBL-induced phototropism.
RESULTS
Expression Analysis of phot1 and phot2 in Arabidopsis Etiolated Hypocotyls
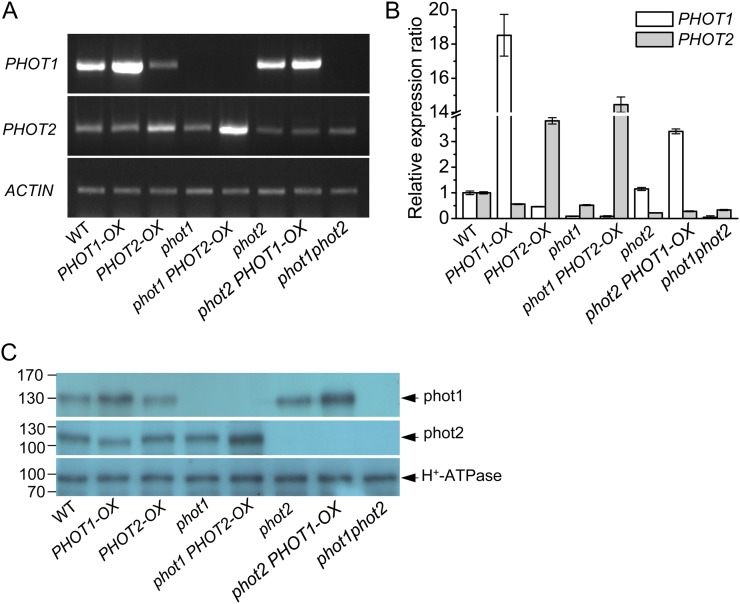
To investigate the functions of the two phototropins (phot1 and phot2) in HBL-induced responses, we transformed the wild type and phot1 and phot2 mutants with PHOT expression constructs driven by the SUPER promoter (Bai et al., 2009). Based on the segregation of hygromycin resistance, the overexpression homozygous lines were isolated for further analysis. Figure 1A shows results after reverse transcription (RT)-PCR analysis of PHOT1 and PHOT2 transcripts in Arabidopsis etiolated hypocotyls. Similar to previous studies in Arabidopsis leaves (Kinoshita et al., 2001), the PHOT1 mRNA was found in the wild type and the phot2 mutant but not in phot1 and phot1phot2 mutants. However, although the transcript level of PHOT2 was lower than that of PHOT1, the PHOT2 mRNA was still found in the wild type, phot1, phot2, and phot1phot2 mutants. Furthermore, the transcript levels of PHOT1 or PHOT2 were enhanced in PHOT1 or PHOT2 overexpression transgenic lines. Consistently, quantitative real-time RT-PCR analysis (Fig. 1B) and immunoblotting detection (Fig. 1C) also showed similar results.
Figure 1.
Analysis of the expression of PHOT1 and PHOT2 genes in the hypocotyl of Arabidopsis etiolated seedlings by RT-PCR, qRT-PCR, and western blotting. A, RT-PCR analysis of PHOT1 and PHOT2 gene expression levels in wild-type (WT), phot1, phot2, and phot1phot2, PHOT1-OX (transgenic PHOT1 in the wild type), PHOT2-OX (transgenic PHOT2 in the wild type), phot1 PHOT2-OX (transgenic PHOT2 in phot1), and phot2 PHOT1-OX (transgenic PHOT1 in phot2) plants. B, qRT-PCR analysis of PHOT1 and PHOT2 gene expression levels in wild-type, mutant, and transgenic plants. C, Western-blot analysis of phot1, phot2, and the plasma membrane H+-ATPase in the hypocotyl of Arabidopsis etiolated seedlings. To determine phot1 and phot2, 40 μg (for phot1) or 60 μg (for phot2) of protein was loaded in each lane. For the plasma membrane H+-ATPase, 40 μg of protein was loaded in each lane. The numbers on the left indicate molecular masses. Experiments conducted three times on different occasions gave similar results. [See online article for color version of this figure.]
HBL Specifically Induces a Transient Increase in Cytosolic Ca2+ in Hypocotyls
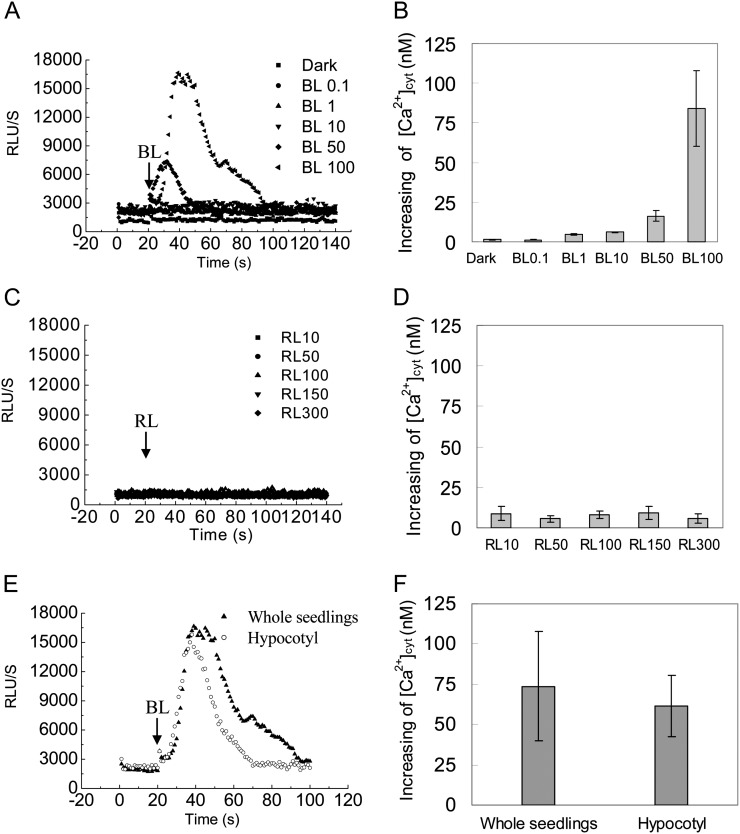
Although calcium plays a role in the process of BL-dependent responses (Harada and Shimazaki, 2007), the nature of changes in cytosolic Ca2+ in etiolated seedling hypocotyls in response to BL remains largely unknown. Here, we made transgenic Arabidopsis that expressed aequorin in wild-type and mutant plants, in which the transgenic aequorin system had been confirmed to work well by the classic cold treatment (Knight et al., 1996; Supplemental Fig. S1). Elevations in average relative luminescence units (RLU) were interpreted as a faithful reporter of increases in [Ca2+]cyt when etiolated seedlings of wild-type plants were irradiated with various fluence rates of BL for 15 s. An instantaneous spike, with a maximum average RLU value of approximately 17,000, was observed, lasting about 80 s before the luminescence returned to the resting level only at HBL (100 μmol m−2 s−1; Fig. 2A). The peak [Ca2+]cyt by HBL was 83.93 ± 23.66 nm, which can be estimated from the aequorin luminescence according to a previous method (Baum et al., 1999; Fig. 2B). In contrast, the lower fluence rates of BL pulses (less than 50 μmol m−2 s−1) had no significant influence on [Ca2+]cyt. To further examine the specificity of HBL-induced transient increases in [Ca2+]cyt, a parallel experiment was performed at various fluence rates of red light. The results show that red light does not induce the transient increases in [Ca2+]cyt (Fig. 2, C and D).
Figure 2.
Changes in cytosolic Ca2+ induced by BL in Arabidopsis etiolated seedlings. Aequorin luminescence is shown as the relative luminescence measured with the Auto Lumat LB9507 Luminometer. A and C, Changes in relative luminescence (RLU) indicate the changes in [Ca2+]cyt in response to different intensities of BL or red light (RL). Etiolated seedlings of the wild type were irradiated with different intensities of BL for 15 s (A) and red light for 15 s (C). B and D, Mean changes in the cytosolic Ca2+ of A and C. E, The changes in relative luminescence in whole seedlings and hypocotyls were induced by irradiation with 100 μmol m−2 s−1 BL for 15 s. F, Mean changes in the cytosolic Ca2+ of E. Values are means of three independent experiments (seven to 21 measurements each) with sd.
To exclude the effects of unexpanded little cotyledons and roots on the increase in [Ca2+]cyt, we compared the trace [Ca2+]cyt and the peak [Ca2+]cyt between the intact etiolated seedling and the decapitated seedling and found that there were no significant differences in changes of [Ca2+]cyt after illumination with a 15-s HBL pulse (Fig. 2, E and F). These results suggest that HBL-induced transient increases of [Ca2+]cyt in intact etiolated seedlings come principally from the hypocotyl sections.
Phototropins Mediate the HBL-Induced Ca2+ Increases in Hypocotyls
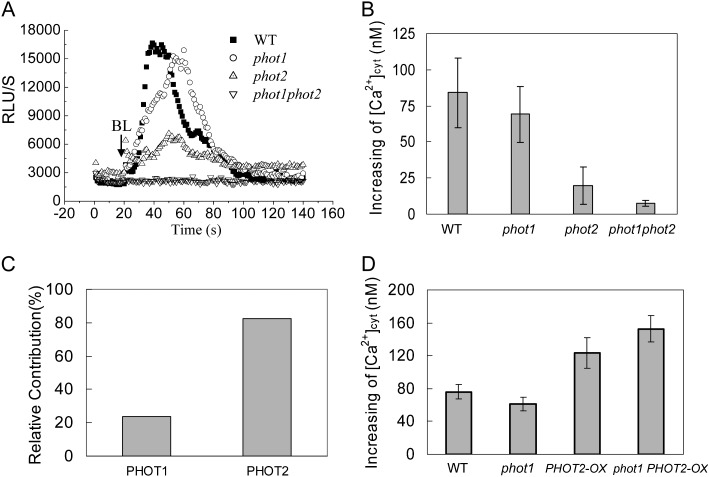
Since BL receptor phototropins mediate various physiological processes in plants (Christie, 2007), we further characterized the significance of phototropins for HBL-induced Ca2+ increases by using phototropin single (phot1 and phot2) and double (phot1phot2) mutants containing transgenic aequorin. When these etiolated seedlings were exposed to HBL at 100 μmol m−2 s−1 for 15 s, the phot1 mutant essentially resembled the wild-type plant, with a significant transient increase of RLU (Fig. 3A), and the peak [Ca2+]cyt was 84.09 ± 23.48 nm in wild-type and 68.94 ± 19.31 nm in phot1 hypocotyls (Fig. 3B). The relative contribution of phot1 (or phot2) was calculated as the percentage difference in HBL-induced increase in [Ca2+]cyt between phot1 (or phot2) and the wild type (Fig. 3C) and showed that the HBL-induced increase in [Ca2+]cyt was primarily attributed to phot2.
Figure 3.
Changes in cytosolic Ca2+ induced by BL in etiolated seedlings of phot1- or phot2-deficient mutants and overexpression plants. A, Changes in relative luminescence (RLU) indicate the changes in [Ca2+]cyt in response to 100 μmol m−2 s−1 BL for 15 s in etiolated seedlings of the wild type (WT), phot1 and phot2 mutants, and phot1phot2 double mutants. B, The mean changes in [Ca2+]cyt shown in A. The values are averages of four independent experiments (11–21 measurements each) with sd. C, Relative contributions of phot1 and phot2 to the BL-induced increases in [Ca2+]cyt. D, The changes in [Ca2+]cyt in response to 100 μmol m−2 s−1 BL for 15 s in 3-d-old wild-type, phot1, PHOT2-OX, and phot1 PHOT2-OX seedlings. The values are averages of three independent experiments (nine to 15 measurements each) with sd.
To further confirm the contribution of phot2 to the increase in [Ca2+]cyt, we also overexpressed the PHOT2 gene by placing its complete coding sequence under the control of the SUPER promoter. As shown in Figure 3D, the overexpression of PHOT2 in homozygous phot1 and wild-type plants drastically enhanced the increase in [Ca2+]cyt, and the role of transgenic PHOT2 in the absence of PHOT1 (phot1 PHOT2-OX) was stronger than that in the presence of PHOT1 (PHOT2-OX), indicating the crucial role of phot2 in regulating the HBL-induced increase in [Ca2+]cyt.
HBL-Induced Ca2+ Increases Are Modulated Differently by phot1 and phot2
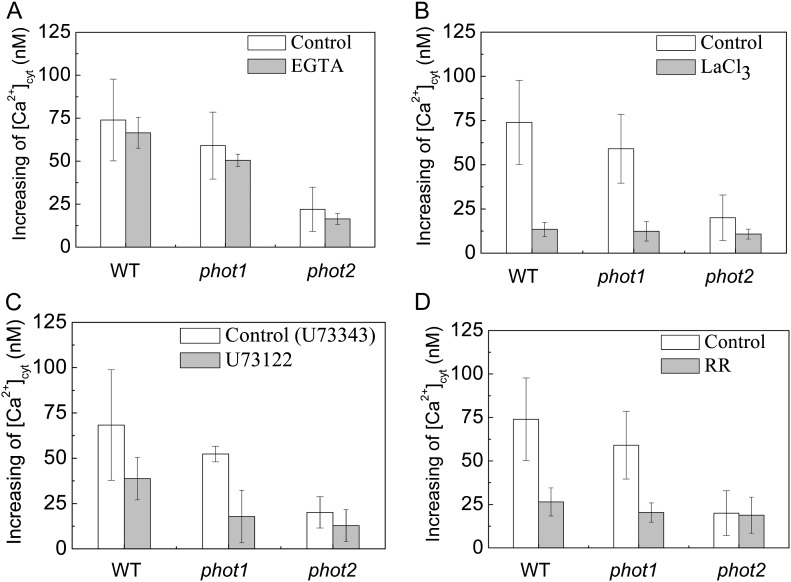
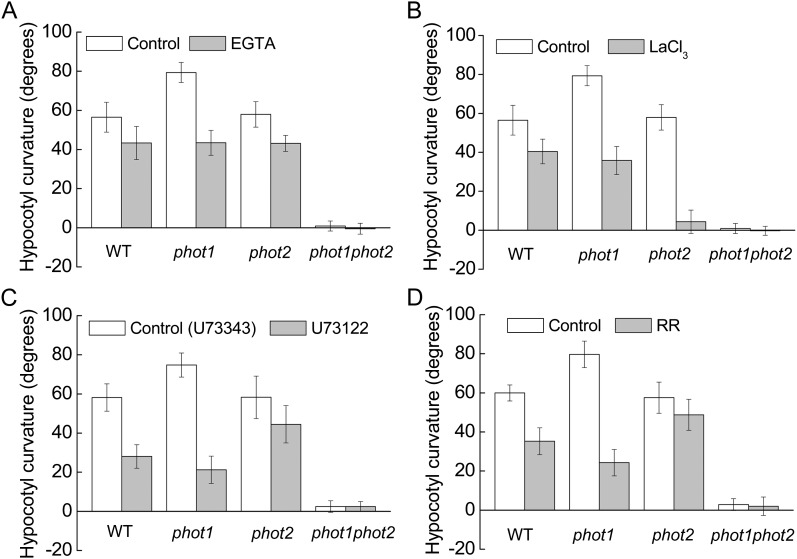
To further analyze the specificity of HBL-induced cytosolic Ca2+ increases, we determined the source of Ca2+ by pharmacological experiments. An extracellular Ca2+ chelating agent, EGTA (2 mm), only slightly reduced the increases in [Ca2+]cyt (Fig. 4A). However, a channel blocker, LaCL3 (1 mm), significantly reduced the increases in [Ca2+]cyt to about 81.2%, 78.8%, and 55.2% of control levels in the wild type and phot1 and phot2 mutants, respectively (Fig. 4B). Considering that LaCl3, the plasma membrane Ca2+ channel blocker identified (Monshausen et al., 2009), can also enter plant cells (Klüsener et al., 1995) and undoubtedly inhibits the intracellular Ca2+ channels in internal Ca2+ stores (e.g. vacuoles and endoplasmic reticulum; Gelli and Blumwald, 1993), we thus infer that the internal Ca2+ stores are probably responsible for HBL-induced increases in [Ca2+]cyt. Indeed, U73122 (10 μm), an inhibitor of PLC, and rutherium red (RR; 20 μm), an endomembrane Ca2+ channel inhibitor, significantly reduced the increases in [Ca2+]cyt in the wild-type plant and the phot1 mutant. However, both inhibitors did not significantly reduced the increase in [Ca2+]cyt in the phot2 mutant (Fig. 4, C and D), which resembled the role of LaCL3 (Fig. 4B). U73122 has been described as inhibiting PLC activity, thus reducing the amount of inositol 1,4,5-triphosphate and blocking inositol 1,4,5-triphosphate-sensitive Ca2+ release from internal stores in plant cells (Harada et al., 2003). These results strongly suggest that phot2 mediates HBL-induced transient increases in [Ca2+]cyt mainly by an inner store-dependent Ca2+-release pathway.
Figure 4.
Effects of various inhibitors on changes in cytosolic Ca2+ induced by BL in the Arabidopsis wild type (WT) and phot1 and phot2 mutants. A single seedling, treated with each inhibitor in the dark for 1 h, was irradiated with 100 μmol m−2 s−1 BL for 15 s. A, Effects of 2 mm EGTA. B, Effects of 1 mm LaCl3. C, Effects of 10 μm U-73122 (10 μm U-73343, an inactive analog, was used as the control). D, Effects of 20 μm RR. Error bars indicate the se of five to 10 independent experiments.
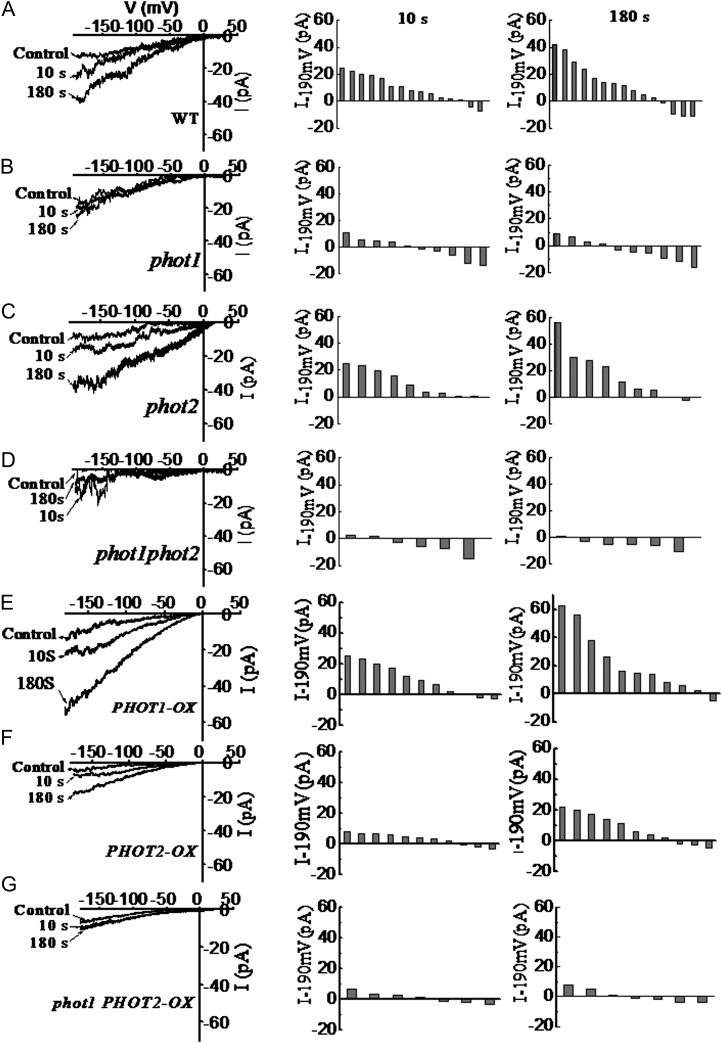
To further detail the source of cytosolic Ca2+, we applied a patch-clamp technique to record the whole-cell Ca2+ currents in Arabidopsis hypocotyl epidermal cell protoplasts in response to a BL pulse (100 μmol m−2 s−1 for 30 s). When the protoplasts were kept in darkness before the experiments and illuminated with dim light during the process of patch-pipette attachment, no significantly pronounced channel activity could be recorded. After the onset of the BL pulse, a time-dependent increase of Ca2+ currents could be monitored in the wild type and the phot2 mutant (Fig. 5, A and C), while the BL pulse failed to induce the increase in Ca2+ currents in phot1 and phot1phot2 mutants (Fig. 5, B and D). Furthermore, overexpression of the PHOT1 gene in the wild-type plant still enhanced the increase in Ca2+ currents (Fig. 5E). By contrast, the overexpression of PHOT2 in the wild-type plant drastically decreased the increase in Ca2+ currents (Fig. 5F), and the overexpression of PHOT2 in homozygous phot1 did not induce any increase in Ca2+ currents (Fig. 5G). These results, together with the data above (Figs. 3 and 4), suggest that phot1 mediates the transient increases in [Ca2+]cyt mainly by activating plasma membrane Ca2+ channels.
Figure 5.
Changes of calcium channel activity induced by BL in hypocotyl epidermal cells of phot1- or phot2-deficient mutants and overexpression plants. At left are current responses of individual cell-attached patches to 1-s voltage ramps from −190 to +30 mV before and after illumination with 100 μmol m−2 s−1 BL for 10 or 180 s. In the middle is channel activation by BL expressed as the current increase within 10 s of 100 μmol m−2 s−1 BL treatment [△I−190mV = (Ibefore − Iafter)]. At right is channel activation by BL expressed as the current increase within 180 s of 100 μmol m−2 s−1 BL treatment [△I−190mV = (Ibefore − Iafter)]. Each bar corresponds to the BL-dependent current difference obtained from one experiment. Current amplitudes were measured at a command voltage of −190 mV and averaged from 10 to 15 ramps. A to G show the changes of calcium channel activity induced by BL in hypocotyl epidermal cells of the wild type (WT), phot1, phot2, phot1phot2, PHOT1-OX, PHOT2-OX, and phot1 PHOT2-OX, respectively.
Phot2 Is Required for Phototropism under HBL
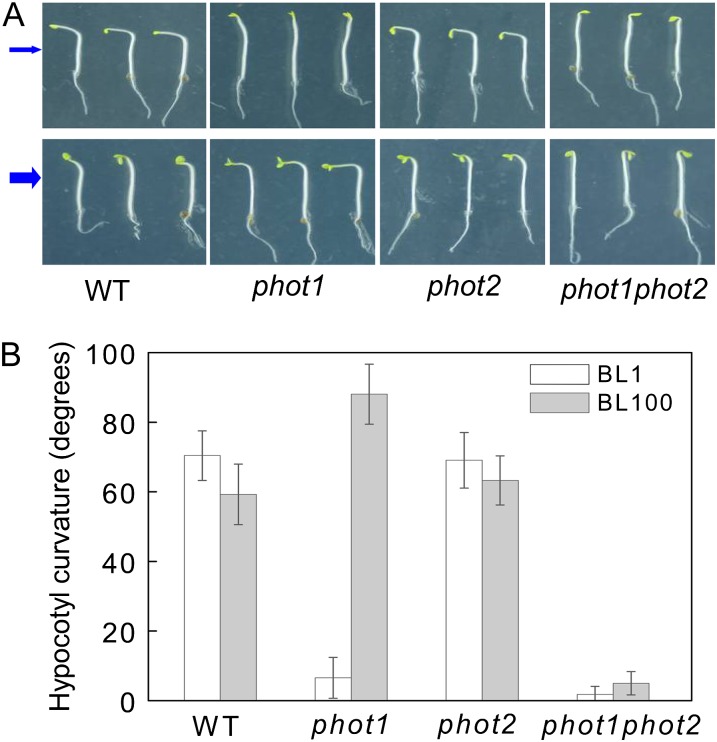
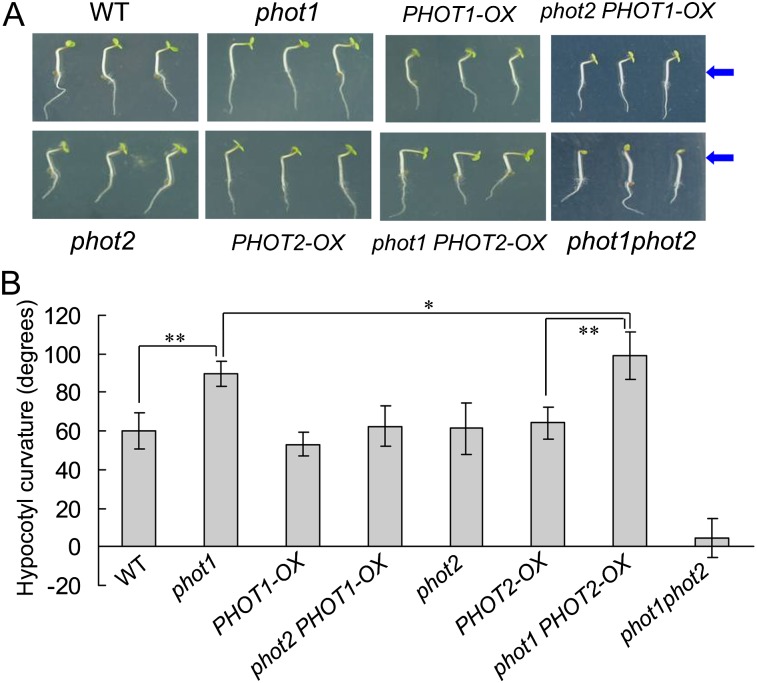
Since HBL can induce the transient increases in [Ca2+]cyt (Fig. 2) and the increases are mainly contributed to phot2 (Fig. 3), the functional roles of phot2-mediated Ca2+ increases were investigated in etiolated seedlings. As shown in Figure 6, the lower fluence rate of BL (1 μmol m−2 s−1) failed to induce the hypocotyl phototropism in phot1 and phot1phot2 mutants. However, the HBL (100 μmol m−2 s−1) significantly induced phototropic bending in phot1 hypocotyls, and the HBL-induced phototropism was stronger in the phot1 mutant than that in the wild-type plant, which indicates that phot2 possibly plays a crucial role in HBL-induced phototropism. To further determine the role of phot2 in phototropism, we constructed a series of PHOT1 and PHOT2 transgenic Arabidopsis lines. The overexpression of PHOT1 in the wild-type plant and the homozygous phot2 mutant showed little role in HBL-induced hypocotyl phototropism, and the mutation of PHOT1 (phot1) and the overexpression of PHOT2 in homozygous phot1 (phot1 PHOT2-OX) significantly enhanced the phototropic responses (Fig. 7).
Figure 6.
Analysis of hypocotyl phototropic curvature in phot1, phot2, and phot1phot2 mutants. A, Photographs were taken of 3-d-old etiolated seedlings of wild-type (WT), phot1, phot2, and phot1phot2 Arabidopsis grown on the same vertical plates and exposed to BL illumination at a fluence rate of 1 μmol m−2 s−1 (top panel) and 100 μmol m−2 s−1 (bottom panel) for 12 h. Arrows indicate the direction of BL illumination. B, Phototropic curvature was measured as the change in hypocotyl angle as determined from an analysis of stacked images. The values are averages of three independent experiments (45–60 measurements each) with sd.
Figure 7.
Analysis of hypocotyl phototropic curvature in phot1- or phot2-deficient mutants and overexpression plants. A, Photographs were taken of 3-d-old wild-type (WT), phot1, phot2, phot1phot2, PHOT1-OX, PHOT2-OX, phot1 PHOT2-OX, and phot2 PHOT1-OX etiolated seedlings grown on the same vertical plates and exposed to BL illumination at 100 μmol m−2 s−1 for 12 h. Arrows indicate the direction of BL illumination. B, Phototropic curvature was measured as the change in hypocotyl angle as determined from an analysis of stacked images. The values are averages of three independent experiments (30–45 measurements each) with sd. *P < 0.05, **P < 0.01.
Considering the regulation of Ca2+-related inhibitors on [Ca2+]cyt (Fig. 4), we next examined the phototropic curvature of the hypocotyls in the presence of these inhibitors. As shown in Figure 8, application of these inhibitors efficiently inhibited HBL-induced hypocotyl phototropism. Compared with EGTA (Fig. 8A), LaCl3 exhibited a stronger inhibition, especially in the phot2 mutant (Fig. 8B), while U73122 and RR showed similar roles in the inhibition of hypocotyl phototropism, with a stronger inhibition in the phot1 mutant (Fig. 8, C and D). These results, together with the data above (Fig. 4), suggest that the transient increases in [Ca2+]cyt are responsible for hypocotyl phototropism in response to HBL and that phot1 and phot2 seem to act in a redundant way, with phot2 playing the major role.
Figure 8.
Effects of various inhibitors on the hypocotyl phototropic curvature induced by BL in the Arabidopsis wild type (WT) and phot1, phot2, and phot1phot2 mutants. Three-day-old etiolated seedlings of wild-type, phot1, phot2, and phot1phot2 Arabidopsis were grown on the same vertical plates and exposed to BL illumination at a fluence rate of 100 μmol m−2 s−1 for 12 h. A, Effects of 2 mm EGTA. B, Effects of 1 mm LaCl3. C, Effects of 10 μm U-73122 (10 μm U-73343, an inactive analog, was used as the control). D, Effects of 20 μm RR. The values are averages of three independent experiments (45–60 measurements each) with sd.
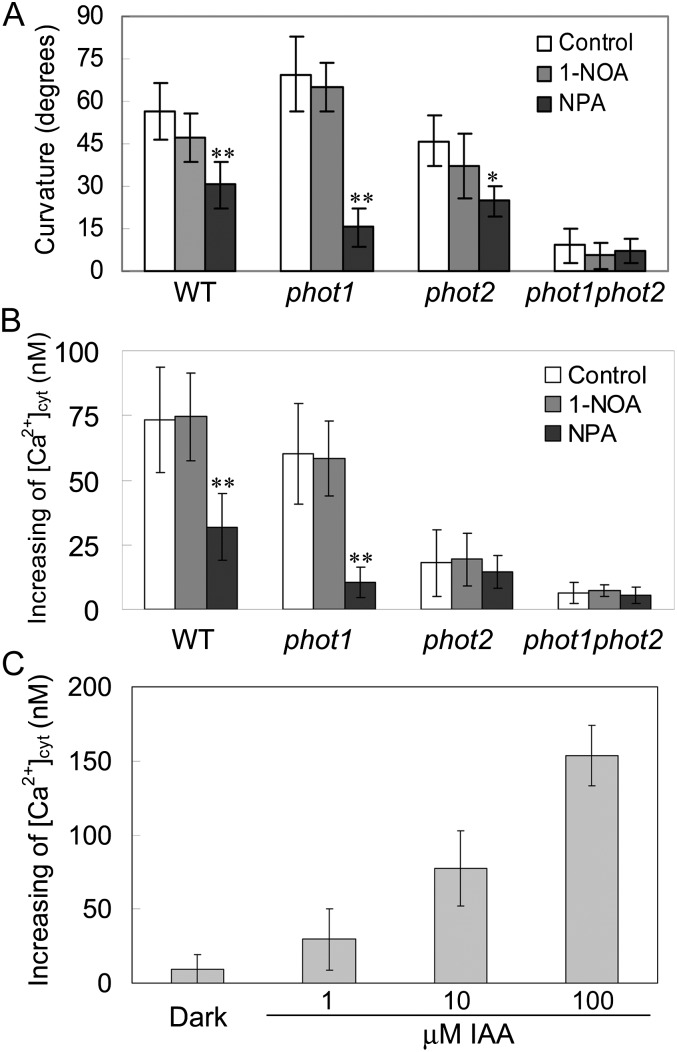
Polar Auxin Transport Is Possibly an Important Event in phot2-Mediated Phototropism and Changes in [Ca2+]cyt
Given the classic Cholodny-Went theory that phototropism is determined by the asymmetric distribution of the phytohormone auxin, we investigated the effects of inhibitors of auxin carriers on phototropism. 1-Naphthoxyacetic acid (1-NOA) at 10 μm, an inhibitor of auxin influx, did not have any effect on HBL-induced phototropic curvatures. By contrast, 1-N-naphthylphthalamic acid (NPA) at 1 μm, an inhibitor of auxin efflux, exhibited a significant inhibition of this phototropism (Fig. 9A). Consistent with the phototropic responses above, NPA also drastically disturbed the HBL-induced increases in [Ca2+]cyt in both the wild type and the phot1 mutant (Fig. 9B). These results suggest that efflux carrier-based polar auxin transport is possibly involved in phot2-dependent responses under HBL.
Figure 9.
Effects of NPA and 1-NOA on hypocotyl phototropic curvature and changes in [Ca2+]cyt induced by BL in phot1, phot2, and phot1phot2 mutants. A, Seedlings were grown vertically on agar plates without (Control) and with 1 μm NPA or 10 μm 1-NOA and then exposed to BL illumination at 100 μmol m−2 s−1 for 12 h. Phototropic curvature was measured as shown in Figure 6B. The values are averages of three independent experiments (40–60 measurements each) with sd. B, Mean changes in cytosolic Ca2+ in response to 100 μmol m−2 s−1 BL for 15 s in etiolated seedlings. Before measuring the [Ca2+]cyt, 1 μm NPA or 10 μm 1-NOA was added to the solution in the dark for 1 h. The values are averages of three independent experiments (11–19 measurements each) with sd. WT, Wild type. C, Effects of exogenous auxin on the changes in [Ca2+]cyt. The values are averages of three independent experiments (10–15 measurements each) with sd. *P < 0.05, **P < 0.01.
Since NPA is shown, like lanthanum, to inhibit the increase in [Ca2+]cyt (Fig. 4B), which implies that the increase of auxin leads to these changes in the hypocotyls, we investigated the effects of exogenous auxin on [Ca2+]cyt in the wild-type plant. As shown in Figure 9C, exogenous auxin application indeed stimulated an increase in [Ca2+]cyt in a dose-dependent manner, which is consistent with early reports (Hasenstein and Evans, 1986; Gehring et al., 1990).
PKS1 Interacts with Phototropins and Their Potential Signaling Elements
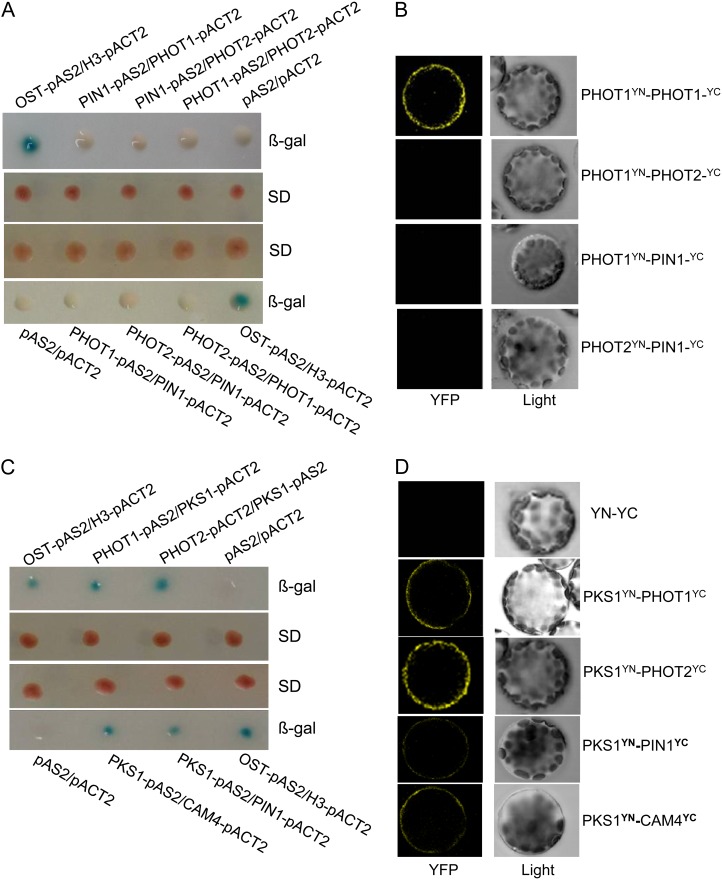
Our genetic results indicate that phot2-dependent calcium signaling is responsible for HBL-induced phototropism and that phot1 and phot2 possibly interact with each other in increasing cytosolic Ca2+. Consequently, we analyzed the interactions between phototropins and their potential signal elements and found that phot1 and phot2 did not interact with each other. Furthermore, PIN1, an auxin efflux carrier identified, was not associated with PHOT1/PHOT2 in vitro (Fig. 10A) and in vivo (Fig. 10B). Given that PKS1 is required for phototropism and associated with PHOT1 in vivo (Lariguet et al., 2006; Boccalandro et al., 2008), we suppose that PKS1 is probably involved in HBL-induced phototropism through interacting with phototropin-related signaling elements (e.g. PIN1 and calmodulins). PKS1 had a direct interaction with PHOT1/PHOT2 in vitro (Fig. 10C) and in vivo (Fig. 10D). Similar interactions were also found between PKS1 and PIN1 or CALMODULIN4 (CAM4; Fig. 10, C and D). Additionally, glutathione S-transferase pull-down assays (Supplemental Materials and Methods) also revealed the direct physical interaction between PKS1 and PHOT1/PHOT2, PIN1, or CAM4 (Supplemental Fig. S2). Taken together, these results indicate that PKS1 may function in coupling phototropin receptor activity to auxin/calcium signaling in HBL-induced phototropism.
Figure 10.
PKS1 interacts with PHOT1, PHOT2, PIN1, or CAM4. A, PIN1 did not interact with PHOT1 or PHOT2, and PHOT1 also did not interact with PHOT2, in the yeast two-hybrid system. Yeast (Saccharomyces cerevisiae strain Y190) containing pAS2-PIN1 or pAS2-PHOT1 as bait and pACT2-PHOT1 or pACT2-PHOT2 as prey, or pAS2-PHOT1 or pAS2-PHOT2 as bait and pACT2-PIN1 or pACT2-PHOT1 as prey, were grown for 48 h on synthetic defined (SD) medium that lacked Trp and Leu (middle panel) and were assayed for LacZ expression by a filter-lift assay for β-galactosidase activity (β-gal; top and bottom panels). The empty vector pAS2 and pACT2 were used as negative controls. pAS2-OST1 and pACT2-H3 were used as positive controls. A blue color indicates interaction. B, PIN1 does not interact with PHOT1 or PHOT2, and PHOT1 also does not interact with PHOT2, in vivo, as determined by BiFC. Left panels show fluorescence images under confocal microscopy, and right panels show bright-field images of the cells. C, PKS1 interacted with PHOT1, PHOT2, PIN1, or CAM4 in the yeast two-hybrid system. Yeast strains containing pAS2-PHOT1 or pAS2-PKS1 as bait and pACT2-PKS1, pACT2-PHOT2, pACT2-PIN1, or pACT2-CAM4 as prey were grown for 48 h on synthetic defined medium that lacked Trp and Leu (middle panel) and were assayed for LacZ expression by a filter-lift assay for β-galactosidase activity (top and bottom panels). D, PKS1 interacted with PHOT1, PHOT2, PIN1, or CAM4 in vivo as determined by BiFC. Left panels show fluorescence images under confocal microscopy, and right panels show bright-field images of the cells. YFP, Yellow fluorescent protein; YN and YC represent pSPYNE and pSPYCE (for split YFP N-terminal/C-terminal fragment expression), respectively.
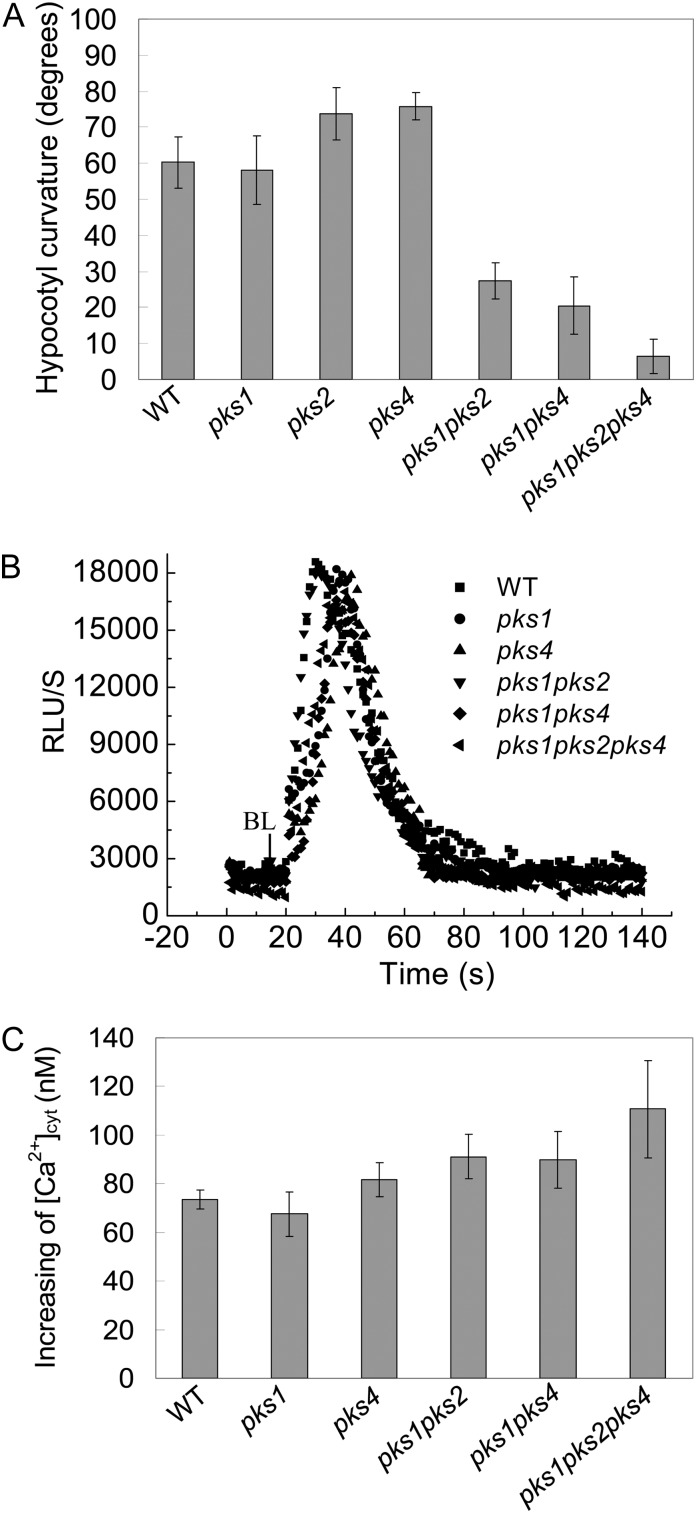
HBL-Induced Phototropic Responses Are Impaired in pks Multiple Mutants
Since there is a possible link between PKS1 and PIN1, as well as calcium signaling components, we thus assessed the functional roles of PKS1 in HBL-induced phototropism by using pks1 and pks multiple mutants. As shown in Figure 11A, HBL-induced phototropic responses were still found in pks single mutants (pks1, pks2, and pks4), while these responses were impaired in pks multiple mutants (pks1pks2, pks1pks4, and pks1pks2pks4), indicating that PKS1, PKS2, and PKS4 have a redundant function in phototropism (Lariguet et al., 2006). In contrast, the HBL-induced increase in [Ca2+]cyt was still found even in pks multiple mutants (Fig. 11, B and C), which suggests that PKSs might function downstream of calcium signals in HBL-induced phototropic responses.
Figure 11.
Analysis of hypocotyl phototropic curvature and changes in cytosolic Ca2+ induced by BL in pks single and multiple mutants. A, Seedlings of the indicated genotypes were grown in darkness for 72 h and treated with 100 μmol m−2 s−1 BL for 12 h. The hypocotyl phototropic curvature was measured as a change in hypocotyl angle as determined from an analysis of stacked images. Data are average curvature angles ± sd with a minimum of 45 seedlings per genotype. B, The changes in relative luminescence (RLU) indicate the changes in [Ca2+]cyt in response to 100 μmol m−2 s−1 BL for 15 s in etiolated seedlings of the indicated genotypes. C, The mean changes in [Ca2+]cyt shown in B. The values are averages of three independent experiments (11–21 measurements each) with sd. WT, Wild type.
DISCUSSION
The Role of phot2 in Hypocotyl Phototropic Responses
This work extends our knowledge of the roles of phot2 in hypocotyl phototropism. On the basis of genetic studies in Arabidopsis, it has become clear that the phototropin family of BL receptors regulates hypocotyl phototropism in a fluence rate-dependent manner. Especially under HBL, the redundant function mediated by both phot1 and phot2 drastically restricts our understanding of the roles of phot2. Since phot1 and phot2 have different photosensitivity for BL-induced phototropism (Sakai et al., 2001) and the light, oxygen, or voltage domains of phot1 and phot2 play different physical roles in phototropin-mediated phototropism (Cho et al., 2007), we infer that the redundant function of phot1 and phot2 is probably attributed to the structure difference between phot1 and phot2. Here, we constructed different genetic lines and checked the effects of BL on hypocotyl curvature in these lines, and we found that phot1 showed a positive phototropic response at HBL (100 μmol m−2 s−1) but no response at the low fluence rate of BL (1 μmol m−2 s−1; Fig. 6). Although the PHOT2 transcript level was lower than the PHOT1 level in etiolated seedlings (Fig. 1), its expression is indeed up-regulated by BL (Kagawa et al., 2001). Moreover, the role of transgenic PHOT2 in the absence of PHOT1 (phot1 PHOT2-OX) was stronger than that in the presence of PHOT1 (PHOT2-OX; Fig. 7), which indicates that phot2 probably predominates in phototropism under high-light conditions and that phot1 negatively regulates the phot2-mediated response through a signal transduction pathway involving Ca2+ (Harada et al., 2003).
Phototropin-Mediated Ca2+ Homeostasis
As a universal second messenger, Ca2+ is involved in diverse cellular functions and is crucial in cell responses to a variety of stimuli. In particular, studies have revealed the importance of cytosolic Ca2+ through phototropins in BL-induced movements such as phototropism, stomatal opening, chloroplast relocation, and solar tracking by leaves (Harada and Shimazaki, 2007). Because of the differences in photosensitivity and redundant function between phot1 and phot2, early research focused on phot1-mediated physiological responses, in which phot1 predominantly mediated the BL-dependent Ca2+ uptake from the apoplast in mesophyll cells (Harada et al., 2003; Stoelzle et al., 2003), deetiolated seedlings (Baum et al., 1999), and etiolated seedlings (Babourina et al., 2002). As for phot2, there are still conflicting reports on the regulation of cytosolic Ca2+ homeostasis, which implies that phot2 may alternatively mediate the Ca2+ influx according to the tissues and light conditions (Harada and Shimazaki, 2007). In this study, we first found that HBL (100 μmol m−2 s−1) specifically induced an increase in [Ca2+]cyt in the etiolated hypocotyls and that the increase in [Ca2+]cyt was attributed primarily to the action of phot2 (Figs. 2 and 3). Moreover, phot2 mediated the Ca2+ uptake mainly from internal calcium stores, not from the apoplast (Figs. 4 and 5), which was different from the previous results obtained in mesophyll cells (Harada et al., 2003). These results demonstrate that phototropins function in different plant organs, possibly through different “Ca2+ signatures.”
A Molecular Link between Cytosolic Ca2+ Changes and Hypocotyl Phototropic Responses
At present, although the increase in [Ca2+]cyt is responsible for the rapid inhibition of hypocotyl growth (Folta et al., 2003), there still are no clues about the link between cytosolic Ca2+ changes and hypocotyl phototropic responses. It is well known that a low fluence rate of blue light (LBL) induces a hypocotyl phototropic response, which is a phot1-dependent process (Inada et al., 2004; Fig. 6). However, LBL did not induce any increase in [Ca2+]cyt (Fig. 2, A and B), indicating that the increase in [Ca2+]cyt is not required for phot1-mediated phototropism under LBL. Furthermore, Folta et al. (2003) have also found that a phot1-mediated transient increase in [Ca2+]cyt is not a signal-transducing event in hypocotyl phototropism. Interestingly, HBL efficiently induced the increase in [Ca2+]cyt (Fig. 3) and hypocotyl phototropism (Figs. 6 and 7), and the application of calcium-related inhibitors significantly inhibited these HBL-induced responses (Figs. 4 and 8), which reveals the novel physical role of the increase in [Ca2+]cyt in HBL-induced phototropism.
The relevance of cytosolic Ca2+ changes and phototropic responses is reminiscent of polar auxin transport. Pharmacological studies showed that NPA, an inhibitor of auxin efflux, significantly inhibited the hypocotyl phototropic responses and the increases in [Ca2+]cyt (Fig. 9), which exhibits the important regulation of auxin transport on cytosolic Ca2+ changes (Hasenstein and Evans, 1986; Gehring et al., 1990). To date, NPA may exert suppressive effects upon the auxin efflux activity of PIN1 (an auxin efflux carrier), which is involved in asymmetric auxin distribution through ABCB19 (an auxin efflux transporter; Sakai and Haga, 2012). However, some lines of evidence have illustrated that changes of cytosolic Ca2+ modulate both auxin transport and PIN polarity (Zhang et al., 2011) and that the mutants impaired in the localization of PIN1 exhibit altered hypocotyl phototropism (Noh et al., 2003). Therefore, the cause-effect relationship between Ca2+ changes and auxin transport in phototropic responses remains an open question. Given the rapid nature of calcium transients, we wondered whether there is a feedback activation cycle between Ca2+ changes and auxin transport in HBL-induced phototropic responses.
The Possible Role of PKS1 in HBL-Induced Phototropic Responses
Since HBL-induced phototropism was stronger in the phot1 mutant than in wild-type plants (Fig. 6) and phot1 possibly inhibited phot2-mediated activity of Ca2+ influx across the plasma membrane in Arabidopsis leaves (Harada et al., 2003), we infer that there are some functional interactions between phot1 and phot2 to mediate the phototropism. However, yeast (Saccharomyces cerevisiae strain Y190) two-hybrid and bimolecular fluorescence complementation (BiFC) assays revealed that the two proteins had no physical interaction and that neither of them interacted with the auxin efflux carrier PIN1, the downstream element of phototropin signaling (Fig. 10, A and B). Recently, the Arabidopsis PKS protein family was reported as a phototropin signal element (de Carbonnel et al., 2010), and auxin transport and the cytosolic Ca2+ changes are responsible for HBL-induced phototropism (Fig. 9). These lines of evidence encouraged us to probe the function of PKS1 during phototropin-dependent phototropism. As expected, PKS1 can interact with PHOT1/PHOT2, PIN1, or calcium-binding proteins such as CAM4 in vitro (Fig. 10C; Supplemental Fig. S2) and in vivo (Fig. 10D). Moreover, the BiFC results not only demonstrated the in vivo interaction of these proteins but also showed the specific localization of the interacting proteins at the plasma membrane (Fig. 10D). In Arabidopsis, there are seven typical CAM genes that encode only four protein isoforms: CAM1/CAM4, CAM2/CAM3/CAM5, CAM6, and CAM7 (McCormack et al., 2005). Intriguingly, PKS1 also interacts with CAM5 and CAM7, as well as CAM4, but not with CAM6 (Supplemental Fig. S3). As Ca2+ sensors, the same or similar CAMs are encoded by multiple CAM genes, but those CAM genes play possibly overlapping but nonidentical roles in regulating diverse downstream targets, leading to a stimulus-appropriate shift in physiology or developmental patterning (DeFalco et al., 2010).
As a phot1-associated protein (Lariguet et al., 2006; Boccalandro et al., 2008), PKS1 appears to play important roles in phototropin-mediated hypocotyl phototropic responses under HBL (Fig. 11A). Nonetheless, whether this is the sole PKS1 action on phot2-mediated responses and what the spatiotemporal relationship among PKS1, PIN1, and CAM4 in phototropin-dependent responses is still remain open. RT-PCR analysis preliminarily showed that the transcript levels of these signal elements have no significant differences among genetic lines except PHOT2-OX (Supplemental Fig. S4). Further work will focus on how phototropins modulate these signal protein activities and their localization in tissue along the etiolated seedling.
MATERIALS AND METHODS
Plant Material, Growth Conditions, and Light Sources
Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 was used as the wild type in this study. The phot1-5, phot2-1, and phot1-5phot2-1 mutant seeds were kindly provided by Ken-ichiro Shimazaki. The pks single and multiple mutant seeds were kindly provided by Christian Fankhauser. For all experiments, seeds were surface sterilized and planted in square petri dishes containing 1.2% agar medium. Seeds were kept in a cold room at 4°C for 3 d and then exposed to white light for 24 h to induce uniform germination. After germination was induced, the petri dishes were incubated vertically to let the seedlings grow on the surface of agar for 3 d in darkness at 21°C to 22°C.
Arabidopsis hypocotyl phototropism was performed as described by Folta et al. (2003) with slight modifications. To measure the phototropic curvature of hypocotyls, we irradiated 3-d-old etiolated seedlings with 1 or 100 μmol m−2 s−1 unilateral BL by using light-emitting diode BL lamps for 12 h. The fluence rate was controlled by filters (film no. 72; Tokyo Butai Shoumei). After irradiation, photographs of the petri dishes were taken. Curvature was measured by using the software e-ruler. Mean values and sd were calculated for each experiment. The significance of differences between wild-type and mutant seedlings was confirmed by Student’s t test.
Transgenic Plants
Aequorin expression vectors cloned into Agrobacterium tumefaciens strain GV3101 were gifts from M.R. Knight. Those constructs were introduced into A. tumefaciens strain LBA4404 and transformed by floral infiltration into Arabidopsis Columbia-0 and the phot1, phot2, phot1phot2, PHOT2-OX, phot1 PHOT2-OX, and pks single and multiple mutants (Harada et al., 2003). Transformants with a 3:1 segregation ratio were self-fertilized, and the homozygous progeny were selected according to resistance to 50 μg mL−1 hygromycin B and identified by PCR with the following aequorin-specific primers: sense primer 5′-ATGACCAGCGAACAATACTCAGT-3′ and antisense primer 5′-TTAGGGGACAGCTCCACCGTAGA-3′. Gene-specific complementary DNA (cDNA) fragments of PHOT1 and PHOT2 were amplified by PCR. Primers were as follows: for PHOT1, sense primer 5′-CCCGACTAGTGATGGAACCAACAGAAAAAC-3′ (SpeI restriction sites underlined) and antisense primer 5′-GGGCGAATTCCTCAAAAAACATTTGTTTGC-3′ (EcoRI restriction sites underlined); for PHOT2, sense primer 5′-GGGTCTAGAAAAGAAACGTTATGGAGAGG-3′ (XbaI restriction sites underlined) and antisense primer 5′-GCCGGTACCGATTAGAAGAGGTCAATGTCC-3′ (KpnI restriction sites underlined). Gene-specific cDNA fragments of PHOT1 or PHOT2 were used for constructing the pBIB vector under the control of the SUPER promoter (Bai et al., 2009). The constructs were introduced into A. tumefaciens strain GV3101 and transformed by floral infiltration into Arabidopsis wild-type, phot1, and phot2 plants.
Total RNA Extraction, RT-PCR, and Quantitative Real-Time PCR Analysis
Total RNA from Arabidopsis seedlings was extracted using TRIzol reagent (Invitrogen). RT was performed using 5 μg of total RNA and SuperScript II Reverse Transcriptase (Invitrogen). The cDNA diluted 10-fold was then used as a template for quantitative real-time (qRT)-PCR amplification. qRT-PCR was performed with the Stratagene M×3005 QPCR system using SYBR Green to monitor double-stranded DNA products.
The primers used for gene expression analysis by semiquantitative RT-PCR are as follows: PHOT1 (forward, 5′-AGAAACCGCAGTGAAAGAG-3′; reverse, 5′-ACGACAACTTGAGCAGCAT-3′); PHOT2 (forward, 5′-ATCGTAACAAGGCTCACCG-3′; reverse, 5′-GTGCCCACCAATCAATAGC-3′); ACTIN2 (forward, 5′-TTCCTCATGCCATCCTCCGTCTT-3′; reverse, 5′-CAGCGATACCTGAGAACATAGTGG-3′). The primers of PKS1, PIN1 and CAM4 used for gene expression analysis by semiquantitative RT-PCR are shown in Supplemental Materials and Methods.
For qRT-PCR analysis, ACTIN2 was used as an internal standard to normalize the data and amplified with the following primer pair: forward, 5′-AACCACTATGTTCTCAGGCATCG-3′; reverse 5′-CCTGGACCTGCCTCATCATACT-3′. The primers used for gene expression analysis by qRT-PCR are as follows: for PHOT1, forward primer 5′-TCTTCTCACGATTGCTCCCAT-3′ and reverse primer 5′-TGCTTGCTCACCTCCACTTGC-3′; for PHOT2, forward primer 5′-CTTCTCACAGTCACTCCTATC-3′ and reverse primer 5′-TTCTCCTTCTGGCGAGCATCAT-3′.
Polyclonal Antibodies and Immunodetection
Polyclonal antibodies raised against the plasma membrane H+-ATPase, phot1, and phot2 were provided by K. Shimazaki and described previously (Kinoshita and Shimazaki, 1999, Doi et al., 2004, Ueno et al., 2005). Immunodetection was performed according to Kinoshita and Shimazaki (1999). Briefly, Arabidopsis etiolated seedling proteins were subjected to SDS-PAGE on an 8.0% gel, and the proteins were transferred to polyvinylidene difluoride membranes (Roche) by electrophoretic transfer. After blocking at room temperature for 2 h, the membranes were incubated with monoclonal anti-H+-ATPase, anti-phot1, or anti-phot2 antibody for 16 h at 4°C. The membranes were then washed three times for 10 min each in Tris-buffered saline and Tween 20, reacted with horseradish peroxidase-conjugated anti-goat IgG secondary antibody, and visualized using Lumi-Light Western Blotting Substrate (Roche) in accordance with the manufacturer’s instructions.
Aequorin Reconstitution and [Ca2+]cyt Measurements
In vivo reconstitution of aequorin from expressed apoaequorin and coelenterazine followed the method of Bai et al. (2009) with slight modifications. A 3-d-old etiolated seedling (about 5–8 mm) grown under dark conditions was floated on 0.1 mL of freshly prepared cp-coelenterazine (a derivative of coelenterazine that has 15 times higher luminescence and faster response time than native coelenterazine; 2.5 μm; Molecular Probes) solution in a 1.5-mL Eppendorf tube for 14 to 20 h in the dark. For pharmacological analysis, tubes were supplemented with each drug 30 to 60 min before BL irradiation. Aequorin luminescence emitted from etiolated seedlings was measured with the Auto Lumat LB9507 Luminometer (Berthold Technologies). All measurements were performed in a dark room at 22°C. Background light (less than 0.01 μmol m−2 s−1) from the computer screen was used for a safelight.
Each seedling containing reconstituted coelenterazine in a 1.5-mL tube was set on the plate holder of the Lumicounter in complete darkness. The basal luminescence level was measured for at least 20 s (five times s−1). Then, the tube was pulled away from the photomultiplier tube of the Lumicounter, the seedling was exposed to BL for 15 s, and the tube was put back to record the aequorin luminescence for 120 s. During the whole process, perturbations should be avoided, which may elicit mechanical signaling. In this way, mechanical stimulation and the time required to put the tube back to the photomultiplier tube were minimal. BL was obtained using cold light-emitting diode BL lamps with a maximum wavelength of 470 nm (lsd). To estimate the Ca2+ concentrations from the luminescence values, 0.1 mL of solution (including 2 m CaCl2 and 20% ethanol) was quickly injected into the tube to discharge all of the remaining aequorin after the experiment. In vivo Ca2+ concentrations were estimated according to the method of Baum et al. (1999). All results were analyzed by means of Student’s t test. In addition, [Ca2+]cyt measurements in response to cold was performed according to Supplemental Materials and Methods.
Patch-Clamp Analysis
Arabidopsis hypocotyl cell protoplasts were isolated according to previous methods (Pandey et al., 2002). The whole-cell voltage-clamp currents of Arabidopsis hypocotyl cells were recorded with an EPC-9 patch-clamp amplifier (HEKA Electronics) as described in a previous report (Miao et al., 2006). To determine the activation state of the channels, 10 repetitive voltage ramps of 1-s duration were applied with an interval of 3 s, after a preactivation period of 50 ms at hyperpolarized potentials, and the current responses were averaged. Voltage protocols are given in the figure legends. Standard solutions contained (in mm) 100 CaCl2, 0.1 dithiothreitol, and 10 MES-Tris (pH 5.6) in the bath and 10 BaCl2, 0.1 dithiothreitol, 4 EGTA, and 10 HEPES-Tris (pH 7.1) in the pipette. d-Sorbitol was used to adjust the osmolarities of bath and pipette solutions to 390 and 420 mmol kg−1, respectively. Pipettes were pulled with a vertical puller (Narishige) modified for two-stage pulls.
For illumination experiments, protoplasts were kept in darkness, and the sealing procedure was done under dim light (2 μmol m−2 s−1 yellow light) provided by the microscope halogen lamp. After establishment of the cell-attached configuration, the light was turned off and background currents were recorded. For subsequent light treatments, BL was generated by a 75-W xenon lamp and directed through a band-pass filter (450–490 nm) of an inverted microscope (Zeiss Axiovert 35). Photon flux density was adjusted to 100 μmol m−2 s−1 by using neutral density filters (Schott). Fluence rates were measured with a quantum meter (Li-250; Li-Cor). PULSEFIT 8.7, IGOR 3.0, and ORIGIN 7.0 software were used for data analysis.
Yeast Two-Hybrid Analysis
For yeast two-hybrid assays, the coding regions of PHOT1, PHOT2, and PKS1 were amplified by PCR with primers that contained appropriate restriction sites. The amplified fragments were inserted into the plasmid pAS2 (Clontech), which contains the GAL4 DNA-binding domain, producing the constructs pAS2-PHOT1, pAS2-PHOT2, pAS2-PIN1, and pAS2-PKS1, which encoded the bait constructs. The full-length coding regions and different fragments of PKS1, PIN1, and CAM4 were cloned in frame between corresponding restriction sites into the pACT2 vector, which contains the GAL4 activation domain, creating the prey plasmids pACT2-PHOT1, pACT2-PHOT2, pACT2-PKS1, pACT2-PIN1, and pACT2-CAM4. Yeast two-hybrid interaction assays were performed as described (Wang et al., 2010). Competent cells of Saccharomyces cerevisiae strain Y190 (Clontech) were transformed simultaneously with pAS2-PHOT1, pAS2-PHOT2, or pAS2-PKS1 and pACT2-PKS1, pACT2-PIN1, or pACT2-CAM4. Yeast (Saccharomyces cerevisiae strain Y190) cells that had been cotransformed with pAS2 and pACT2 with no inserts were used as negative controls, whereas those cotransformed with pAS2-OST1 (for opening stoma transcription factor1) and pACT2-HSF3 (for heat shock protein factor3) were used as positive controls.
BiFC Assay
To measure in vivo interactions, the coding regions of PKS1, PHOT1, PHOT2, PIN1, and CAM4 were amplified by PCR with primers that contained appropriate restriction sites, and the amplified fragments were inserted into the plasmids pSPYNE and pSPYCE, which contain DNA encoding the N-terminal and C-terminal regions of yellow fluorescent protein, respectively (Wang et al., 2010), to form pSPYNE-PKS1, pSPYCE-PHOT1, pSPYCE-PHOT2, pSPYCE-PIN1, and pSPYCE-CAM4, respectively. Protoplasts isolated from Arabidopsis leaves were transformed with the following combinations of plasmids: pSPYNE-PKS1 and pSPYCE-PHOT1, pSPYCE-PHOT2, pSPYCE-PIN1, or pSPYCE-CAM4, according to previous protocols (Wang et al., 2010). Coexpression of pSPYNE-PHOT1 and pSPYCE-PHOT1 as positive controls was as described (Kaiserli et al., 2009). After incubation for 16 to 20 h, the fluorescence of the protoplasts was measured with a confocal laser scanning microscope (FV1000; Olympus). All figures show representative images from three independent experiments.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers PHOT1 (At3g45780), PHOT2 (At5g58140), PKS1 (At2g02950), PIN1 (At1g73590), and CAM4 (At1g66410).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Changes in cytosolic Ca2+ induced by cold in Arabidopsis etiolated seedlings.
Supplemental Figure S2. PKS1 interacts with PIN1, CAM4, PHOT1 and PHOT2 by GST pull-down assays.
Supplemental Figure S3. PKS1 interacts with CAM5 and CAM7.
Supplemental Figure S4. Analysis of the expression of PKS1, PIN1, and CAM4 genes in the hypocotyl of Arabidopsis etiolated seedlings by RT-PCR.
Supplementary Material
Acknowledgments
We thank Ken-ichiro Shimazaki (Kyushu University) for providing the phot1, phot2, and phot1phot2 mutants and polyclonal antibodies, Marc R. Knight (University of Cambridge) for providing aequorin expression vectors cloned into A. tumefaciens strain GV3101, and Christian Fankhauser (University of Geneva) for providing pks multiple mutants.
Glossary
- BL
blue light
- HBL
high fluence rates of blue light
- [Ca2+]cyt
cytosolic Ca2+ concentration
- RT
reverse transcription
- RLU
relative luminescence units
- RR
rutherium red
- 1-NOA
1-naphthoxyacetic acid
- NPA
1-N-naphthylphthalamic acid
- LBL
low fluence rate of blue light
- BiFC
bimolecular fluorescence complementation
- cDNA
complementary DNA
- qRT
quantitative real-time
- PLC
phospholipase C
References
- Babourina O, Godfrey L, Voltchanskii K. (2004) Changes in ion fluxes during phototropic bending of etiolated oat coleoptiles. Ann Bot (Lond) 94: 187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babourina O, Newman I, Shabala S. (2002) Blue light-induced kinetics of H+ and Ca2+ fluxes in etiolated wild-type and phototropin-mutant Arabidopsis seedlings. Proc Natl Acad Sci USA 99: 2433–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Zhang G, Zhou Y, Zhang ZP, Wang W, Du YY, Wu ZY, Song CP. (2009) Plasma membrane-associated proline-rich extensin-like receptor kinase 4, a novel regulator of Ca signalling, is required for abscisic acid responses in Arabidopsis thaliana. Plant J 60: 314–327 [DOI] [PubMed] [Google Scholar]
- Baum G, Long JC, Jenkins GI, Trewavas AJ. (1999) Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+. Proc Natl Acad Sci USA 96: 13554–13559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamins R, Ampudia CS, Hooykaas PJ, Offringa R. (2003) PINOID-mediated signaling involves calcium-binding proteins. Plant Physiol 132: 1623–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeslee JJ, Bandyopadhyay A, Peer WA, Makam SN, Murphy AS. (2004) Relocalization of the PIN1 auxin efflux facilitator plays a role in phototropic responses. Plant Physiol 134: 28–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccalandro HE, De Simone SN, Bergmann-Honsberger A, Schepens I, Fankhauser C, Casal JJ. (2008) PHYTOCHROME KINASE SUBSTRATE1 regulates root phototropism and gravitropism. Plant Physiol 146: 108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Tseng TS, Kaiserli E, Sullivan S, Christie JM, Briggs WR. (2007) Physiological roles of the light, oxygen, or voltage domains of phototropin 1 and phototropin 2 in Arabidopsis. Plant Physiol 143: 517–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM. (2007) Phototropin blue-light receptors. Annu Rev Plant Biol 58: 21–45 [DOI] [PubMed] [Google Scholar]
- Christie JM, Yang H, Richter GL, Sullivan S, Thomson CE, Lin J, Titapiwatanakun B, Ennis M, Kaiserli E, Lee OR, et al. (2011) phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism. PLoS Biol 9: e1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carbonnel M, Davis P, Roelfsema MRC, Inoue S, Schepens I, Lariguet P, Geisler M, Shimazaki K, Hangarter R, Fankhauser C. (2010) The Arabidopsis PHYTOCHROME KINASE SUBSTRATE2 protein is a phototropin signaling element that regulates leaf flattening and leaf positioning. Plant Physiol 152: 1391–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco TA, Bender KW, Snedden WA. (2010) Breaking the code: Ca2+ sensors in plant signalling. Biochem J 425: 27–40 [DOI] [PubMed] [Google Scholar]
- Doi M, Shigenaga A, Emi T, Kinoshita T, Shimazaki K. (2004) A transgene encoding a blue-light receptor, phot1, restores blue-light responses in the Arabidopsis phot1 phot2 double mutant. J Exp Bot 55: 517–523 [DOI] [PubMed] [Google Scholar]
- Folta KM, Lieg EJ, Durham T, Spalding EP. (2003) Primary inhibition of hypocotyl growth and phototropism depend differently on phototropin-mediated increases in cytoplasmic calcium induced by blue light. Plant Physiol 133: 1464–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, Benjamins R, Ouwerkerk PBF, Ljung K, Sandberg G, et al. (2004) A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306: 862–865 [DOI] [PubMed] [Google Scholar]
- Gehring CA, Irving HR, Parish RW. (1990) Effects of auxin and abscisic acid on cytosolic calcium and pH in plant cells. Proc Natl Acad Sci USA 87: 9645–9649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelli A, Blumwald E. (1993) Calcium retrieval from vacuolar pools: characterization of a vacuolar calcium channel. Plant Physiol 102: 1139–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Sakai T, Okada K. (2003) Phot1 and phot2 mediate blue light-induced transient increases in cytosolic Ca2+ differently in Arabidopsis leaves. Proc Natl Acad Sci USA 100: 8583–8588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Shimazaki K. (2007) Phototropins and blue light-dependent calcium signaling in higher plants. Photochem Photobiol 83: 102–111 [DOI] [PubMed] [Google Scholar]
- Harada A, Takemiya A, Inoue S, Sakai T, Shimazaki K. (2013) Role of RPT2 in leaf positioning and flattening and a possible inhibition of phot2 signaling by phot1. Plant Cell Physiol 54: 36–47 [DOI] [PubMed] [Google Scholar]
- Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K, Watahiki MK, Yamamoto K, Liscum E. (2000) The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 12: 757–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstein KH, Evans ML. (1986) Calcium dependence of rapid auxin action in maize roots. Plant Physiol 81: 439–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada S, Ohgishi M, Mayama T, Okada K, Sakai T. (2004) RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin 1 in Arabidopsis thaliana. Plant Cell 16: 887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Takemiya A, Shimazaki K. (2010) Phototropin signaling and stomatal opening as a model case. Curr Opin Plant Biol 13: 587–593 [DOI] [PubMed] [Google Scholar]
- Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M. (2001) Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138–2141 [DOI] [PubMed] [Google Scholar]
- Kaiserli E, Sullivan S, Jones MA, Feeney KA, Christie JM. (2009) Domain swapping to assess the mechanistic basis of Arabidopsis phototropin 1 receptor kinase activation and endocytosis by blue light. Plant Cell 21: 3226–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Kagawa T. (2006) Phototropin and light-signaling in phototropism. Curr Opin Plant Biol 9: 503–508 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K. (2001) Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki Ki. (1999) Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J 18: 5548–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüsener B, Boheim G, Liss H, Engelberth J, Weiler EW (1995) Gadolinium-sensitive, voltage-dependent calcium release channels in the endoplasmic reticulum of a higher plant mechanoreceptor organ. EMBO J 14: 2708–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. (1996) Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8: 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariguet P, Schepens I, Hodgson D, Pedmale UV, Trevisan M, Kami C, de Carbonnel M, Alonso JM, Ecker JR, Liscum E, et al. (2006) PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. Proc Natl Acad Sci USA 103: 10134–10139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack E, Tsai YC, Braam J. (2005) Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci 10: 383–389 [DOI] [PubMed] [Google Scholar]
- Miao YC, Lv D, Wang PC, Wang XC, Chen J, Miao C, Song CP. (2006) An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 18: 2749–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Bibikova TN, Weisenseel MH, Gilroy S. (2009) Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell 21: 2341–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motchoulski A, Liscum E. (1999) Arabidopsis NPH3: a NPH1 photoreceptor-interacting protein essential for phototropism. Science 286: 961–964 [DOI] [PubMed] [Google Scholar]
- Noh B, Bandyopadhyay A, Peer WA, Spalding EP, Murphy AS. (2003) Enhanced gravi- and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature 423: 999–1002 [DOI] [PubMed] [Google Scholar]
- Pandey S, Wang XQ, Coursol SA, Assmann SM. (2002) Preparation and applications of Arabidopsis thaliana guard cell protoplasts. New Phytol 153: 517–526 [DOI] [PubMed] [Google Scholar]
- Sakai T, Haga K. (2012) Molecular genetic analysis of phototropism in Arabidopsis. Plant Cell Physiol 53: 1517–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K. (2001) Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98: 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Wada T, Ishiguro S, Okada K. (2000) RPT2: a signal transducer of the phototropic response in Arabidopsis. Plant Cell 12: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Briggs WR. (2002) Cellular and subcellular localization of phototropin 1. Plant Cell 14: 1723–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoelzle S, Kagawa T, Wada M, Hedrich R, Dietrich P. (2003) Blue light activates calcium-permeable channels in Arabidopsis mesophyll cells via the phototropin signaling pathway. Proc Natl Acad Sci USA 100: 1456–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu N, Kagawa T, Wada M. (2005) An auxilin-like J-domain protein, JAC1, regulates phototropin-mediated chloroplast movement in Arabidopsis. Plant Physiol 139: 151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng TS, Briggs WR. (2010) The Arabidopsis rcn1-1 mutation impairs dephosphorylation of Phot2, resulting in enhanced blue light responses. Plant Cell 22: 392–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K, Kinoshita T, Inoue S, Emi T, Shimazaki K. (2005) Biochemical characterization of plasma membrane H+-ATPase activation in guard cell protoplasts of Arabidopsis thaliana in response to blue light. Plant Cell Physiol 46: 955–963 [DOI] [PubMed] [Google Scholar]
- Wang PC, Du YY, Li Y, Ren DT, Song CP. (2010) Hydrogen peroxide-mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell 22: 2981–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Vanneste S, Brewer PB, Michniewicz M, Grones P, Kleine-Vehn J, Löfke C, Teichmann T, Bielach A, Cannoot B, et al. (2011) Inositol trisphosphate-induced Ca2+ signaling modulates auxin transport and PIN polarity. Dev Cell 20: 855–866 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.