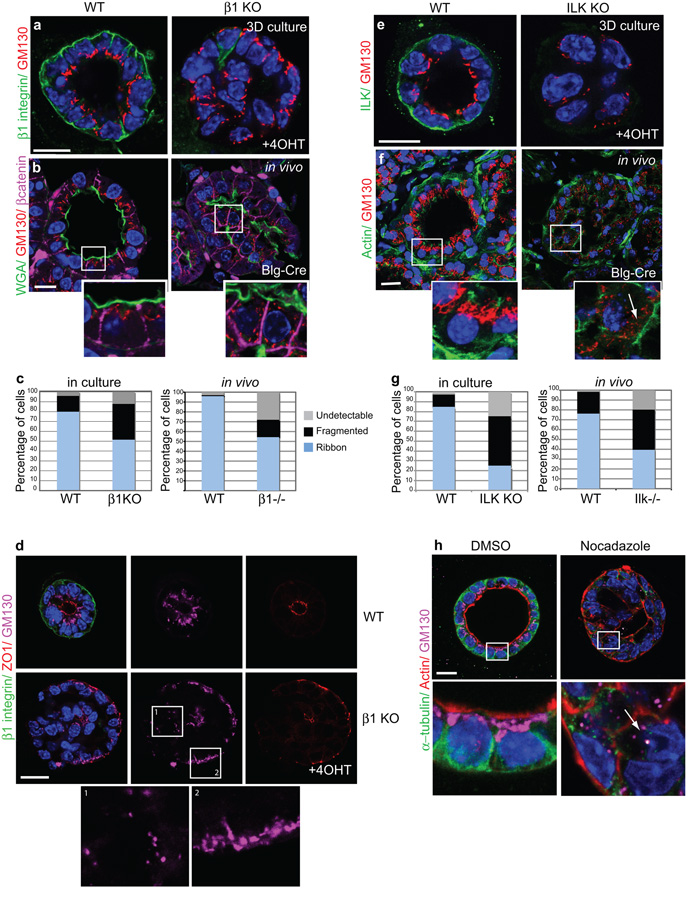
Figure 4. β1 integrins and ILK control internal Golgi polarity.
(a) WT and β1-KO cultured acini, stained for β1-integrin and GM130.
(b) WT and β1−/− in vivo acini, stained for WGA488, GM130 and β-catenin. Note the repositioning or fragmentation of Golgi following β1-integrin deletion. Bar: 15μm.
(c) Histograms represents average Golgi positioning (%) in β1fx/fx:CreER™ cultured acini or from 9 areas imaged within each β1fx/fx:Blg-Cre gland: ‘Ribbon’ Golgi are those accumulated halfway perinuclear towards the apical surface, but they are ‘Fragmented’ if localized more than half way around the nucleus. In some cells Golgi were undetectable by GM130 immunostaining and scored as ‘Undetectable’.
(d) WT and β1-KO acini stained for β1-integrin, ZO1 and GM130. Note that regions of inverted polarity and no polarity are found in the same β1-KO. In these cases, ribbon Golgi distribution to peripheral edges in β1-KO acini correlates with intact apical polarity (box 2) whilst fragmented Golgi are evident in cells that have lost apical polarity (box 1). Bar: 10 μm
(e,f) GM130 staining shows sub-apical Golgi in WT (e) cultured and (f) in vivo acini and fragmentation upon ILK depletion. Arrow indicates fragmented Golgi. Bar: 15μm.
(g) Histogram represents average Golgi positioning (%) in Ilkfx/fx:CreER™ cultured acini or from 9 areas imaged within each Ilkfx/fx:Blg-Cre gland (as in Fig 4b).
(h) ICR acini, treated with DMSO or Nocodazole (24h). Arrow indicates Golgi dispersal after MT disruption. Bar: 10μm.