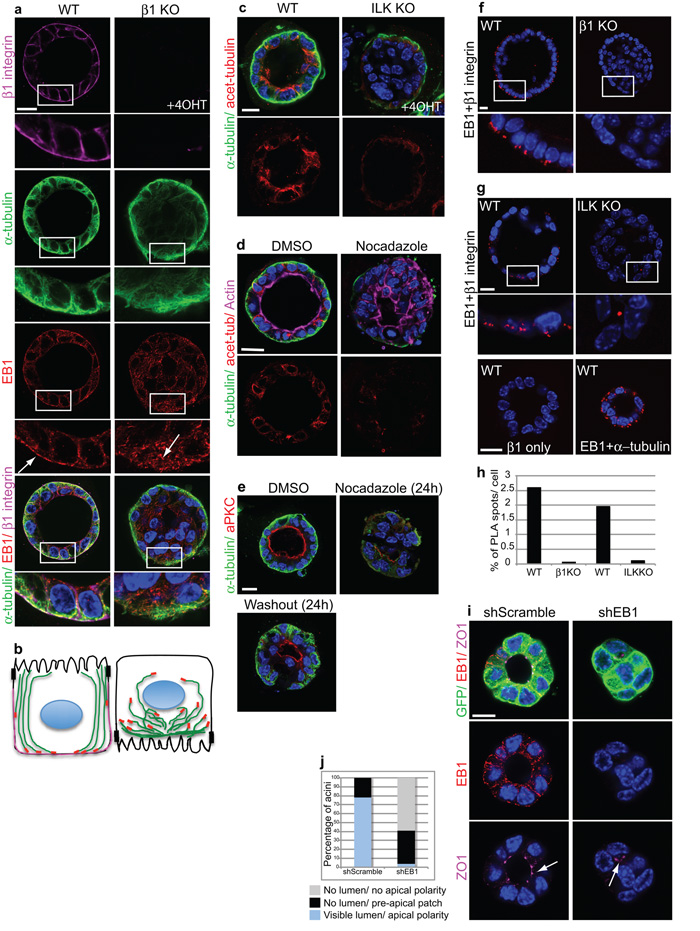
Figure 5. β1-integrins and ILK control polarity and lumens through polarization of microtubules.
(a) WT and β1-KO acini stained for α-tubulin, EB1 and β1-integrin. Integrin deletion prevented plus-end MT orientation and alignment along the apicobasal polarity axis. Bar: 10 μm.
(b) Schematic of (a) showing MT orientation (green) and location of EB1 (red) and β1 integrin (magenta) in WT and β1-KO acini.
(c) WT and ILK-KO acini stained for α-tubulin and acetylated α-tubulin. Bar 10μm.
(d) Polarized ICR MEC acini were treated with DMSO or Nocodazole (1.5h), fixed and stained with antibodies to α-tubulin, acetylated tubulin and Alexa 647 phalloidin. Bar: 10μm.
(e) Polarized ICR acini were treated with DMSO or Nocodazole (24h), then either harvested or the drug was washed out and cells cultured for a further 24 h. MT disruption depolarized acini, but polarity was rescued after the washout. Bar: 10μm.
(f,g) WT and β1-KO (f), or ILK-KO (g) acini were stained with β1 integrin and EB1 antibodies, followed by proximity ligation assay (PLA) to detect complex formation between these two proteins. Each PLA spot represents a point of interaction between β1-integrin and EB1. Note in the absence of ILK, β1 integrin-EB1 complexes do not form. EB1 and α-tubulin were used as a positive control for interaction, showing PLA spots throughout the cells. β1-integrin alone was used as a negative control for the PLA. Bar: 10μm.
(h) Histogram represents the average number (%) of PLA spots per cell within acini.
(i) ShRNA lentiviral knock down of EB1 in MECs resulted in abnormal lumens. Arrow indicates a visible lumen with apical ZO1 in shScrambled acini but only a small pre-apical patch and no lumen in shEB1 acini. Bar: 7μm
(j) Quantification of scrambled or EB1 knockdown acini with polarized lumens.
See also Supplementary Figs. 5, 6.