Abstract
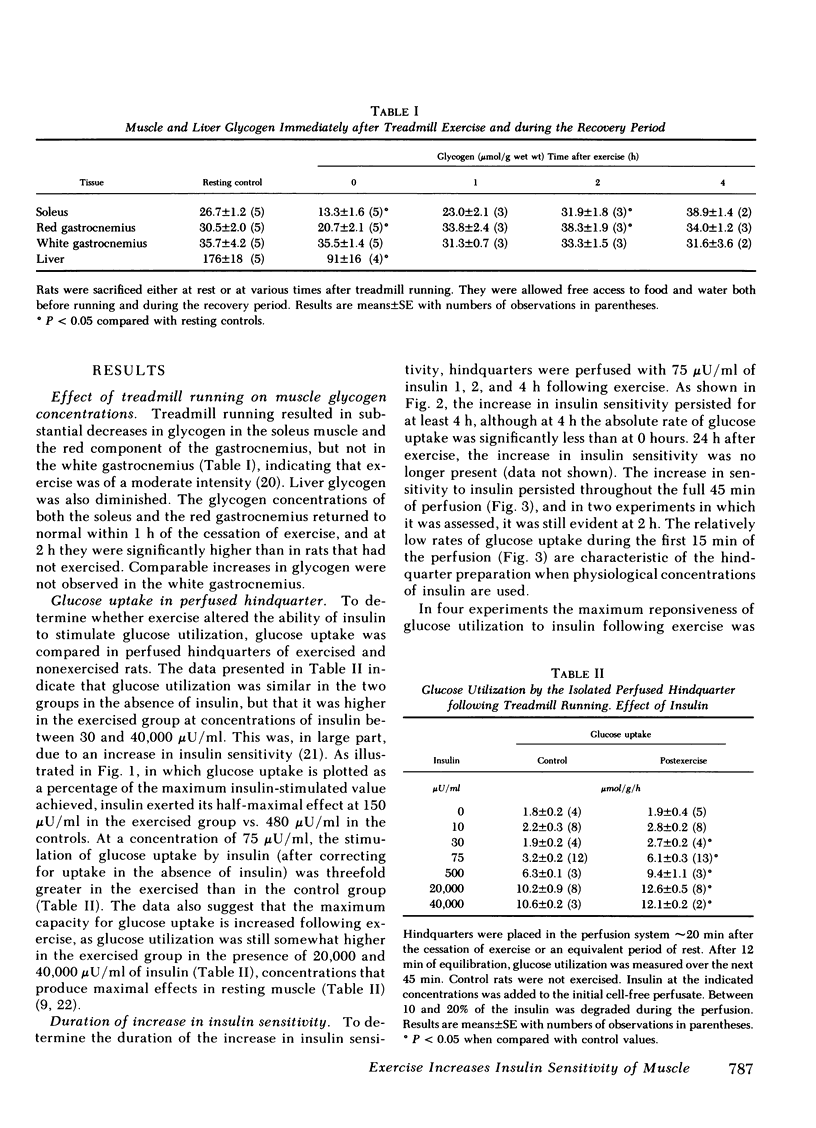
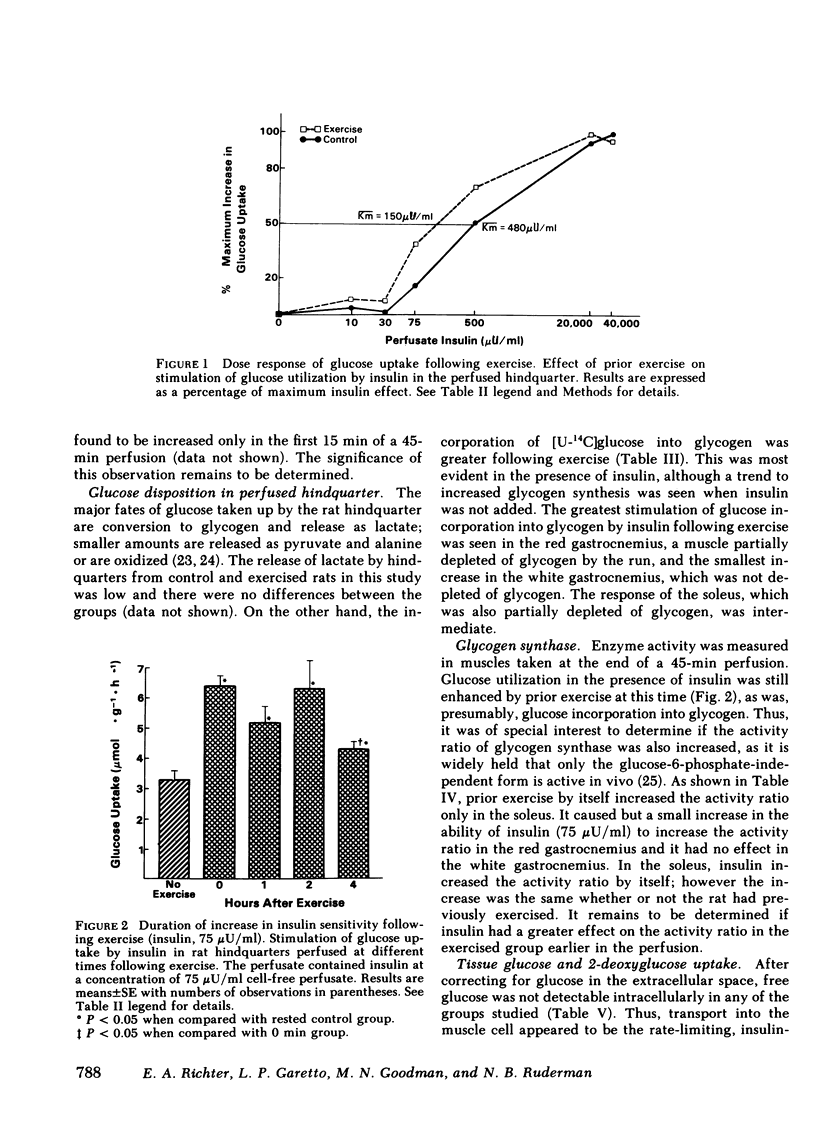
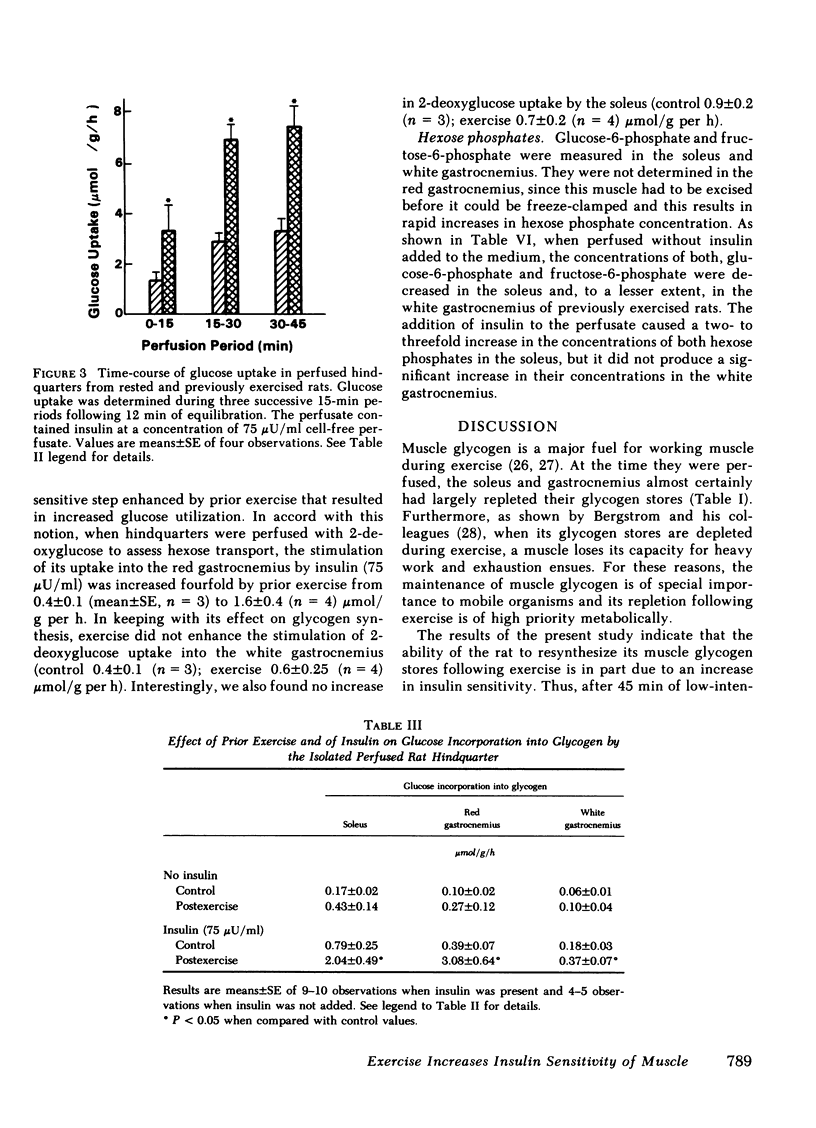
Muscle glycogen stores are depleted during exercise and are rapidly repleted during the recovery period. To investigate the mechanism for this phenomenon, untrained male rats were run for 45 min on a motor-driven treadmill and the ability of their muscles to utilize glucose was then assessed during perfusion of their isolated hindquarters. Glucose utilization by the hindquarter was the same in exercised and control rats perfused in the absence of added insulin; however, when insulin (30-40,000 μU/ml) was added to the perfusate, glucose utilization was greater after exercise. Prior exercise lowered both, the concentration of insulin that half-maximally stimulated glucose utilization (exercise, 150 μU/ml; control, 480 μU/ml) and modestly increased its maximum effect. The increase in insulin sensitivity persisted for 4 h following exercise, but was not present after 24 h. The rate-limiting step in glucose utilization enhanced by prior exercise appeared to be glucose transport across the cell membrane, as in neither control nor exercised rats did free glucose accumulate in the muscle cell.
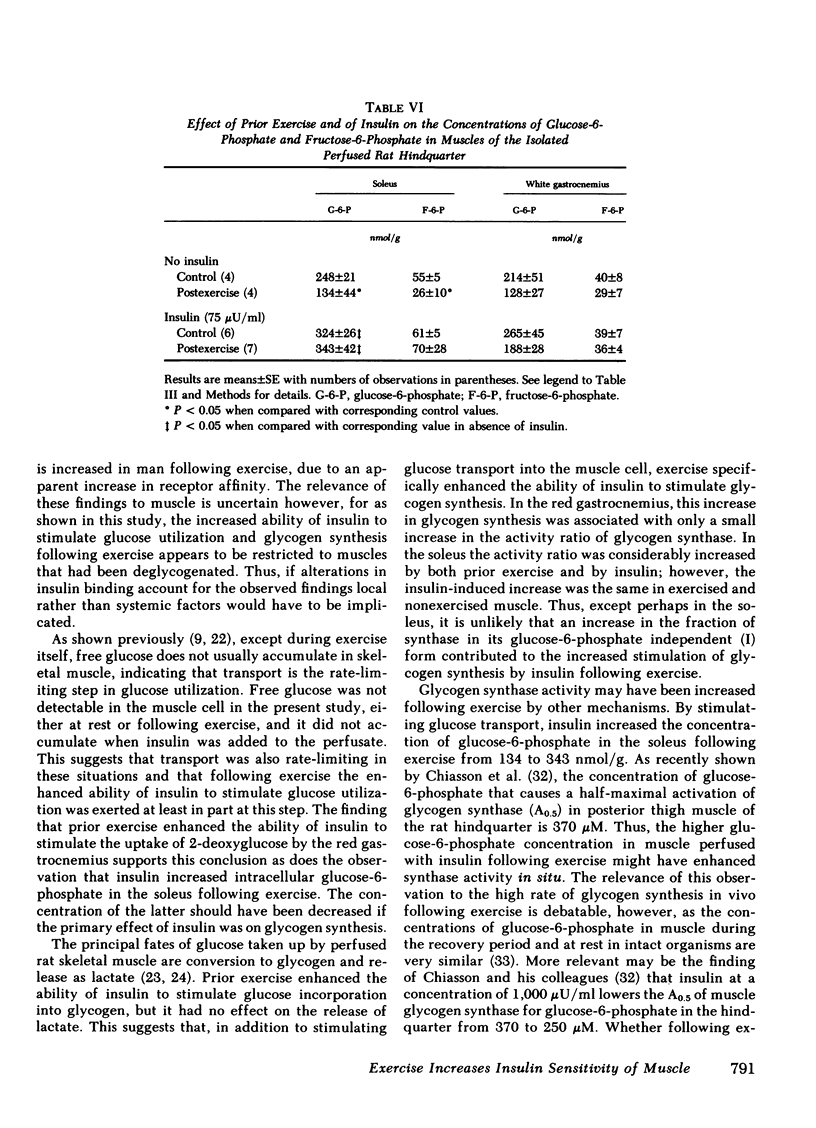
Following exercise, the ability of insulin to stimulate the release of lactate into the perfusate was unaltered; however its ability to stimulate the incorporation of [14C]glucose into glycogen in certain muscles was enhanced. Thus at a concentration of 75 μU/ml insulin stimulated glycogen synthesis eightfold more in the fast-twitch red fibers of the red gastrocnemius than it did in the same muscle of nonexercised rats. In contrast, insulin only minimally increased glycogen synthesis in the fast-twitch white fibers of the gastrocnemius, which were not glycogen-depleted. The uptake of 2-deoxyglucose by these muscles followed a similar pattern suggesting that glucose transport was also differentially enhanced. Prior exercise did not enhance the ability of insulin to convert glycogen synthase from its glucose-6-phosphate-dependent (D) to its glucose-6-phosphate-independent (1) form. On the other hand, following exercise, insulin prevented a marked decrease in muscle glucose-6-phosphate, which could have diminished synthase activity in situ. The possibility that exercise enhanced the ability of insulin to convert glycogen synthase D to an intermediate form of the enzyme, more sensitive to glucose-6-phosphate, remains to be explored.
These results suggest that following exercise, glucose transport and glycogen synthesis in skeletal muscle are enhanced due at least in part to an increase in insulin sensitivity. They also suggest that this increase in insulin sensitivity occurs predominantly in muscle fibers that are deglycogenated during exercise.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Armstrong R. B., Saubert C. W., 4th, Sembrowich W. L., Shepherd R. E., Gollnick P. D. Glycogen depletion in rat skeletal muscle fibers at different intensities and durations of exercise. Pflugers Arch. 1974;352(3):243–256. doi: 10.1007/BF00590489. [DOI] [PubMed] [Google Scholar]
- Berger M., Hagg S. A., Goodman M. N., Ruderman N. B. Glucose metabolism in perfused skeletal muscle. Effects of starvation, diabetes, fatty acids, acetoacetate, insulin and exercise on glucose uptake and disposition. Biochem J. 1976 Aug 15;158(2):191–202. doi: 10.1042/bj1580191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., Hagg S., Ruderman N. B. Glucose metabolism in perfused skeletal muscle. Interaction of insulin and exercise on glucose uptake. Biochem J. 1975 Jan;146(1):231–238. doi: 10.1042/bj1460231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström J., Hermansen L., Hultman E., Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand. 1967 Oct-Nov;71(2):140–150. doi: 10.1111/j.1748-1716.1967.tb03720.x. [DOI] [PubMed] [Google Scholar]
- Brady L. J., Goodman M. N., Kalish F. N., Ruderman N. B. Insulin binding and sensitivity in rat skeletal muscle: effect of starvation. Am J Physiol. 1981 Feb;240(2):E184–E190. doi: 10.1152/ajpendo.1981.240.2.E184. [DOI] [PubMed] [Google Scholar]
- Chiasson J. L., Dietz M. R., Shikama H., Wootten M., Exton J. H. Insulin regulation of skeletal muscle glycogen metabolism. Am J Physiol. 1980 Jul;239(1):E69–E74. doi: 10.1152/ajpendo.1980.239.1.E69. [DOI] [PubMed] [Google Scholar]
- Conlee R. K., Hickson R. C., Winder W. W., Hagberg J. M., Holloszy J. O. Regulation of glycogen resynthesis in muscles of rats following exercise. Am J Physiol. 1978 Sep;235(3):R145–R150. doi: 10.1152/ajpregu.1978.235.3.R145. [DOI] [PubMed] [Google Scholar]
- Costill D. L., Cleary P., Fink W. J., Foster C., Ivy J. L., Witzmann F. Training adaptations in skeletal muscle of juvenile diabetics. Diabetes. 1979 Sep;28(9):818–822. doi: 10.2337/diab.28.9.818. [DOI] [PubMed] [Google Scholar]
- Fell R. D., McLane J. A., Winder W. W., Holloszy J. O. Preferential resynthesis of muscle glycogen in fasting rats after exhausting exercise. Am J Physiol. 1980 May;238(5):R328–R332. doi: 10.1152/ajpregu.1980.238.5.R328. [DOI] [PubMed] [Google Scholar]
- Goodman M. N., Berger M., Ruderman N. B. Glucose metabolism in rat skeletal muscle at rest. Effect of starvation, diabetes, ketone bodies and free fatty acids. Diabetes. 1974 Nov;23(11):881–888. doi: 10.2337/diab.23.11.881. [DOI] [PubMed] [Google Scholar]
- Goodman M. N., Ruderman N. B., Aoki T. T. Glucose and amino acid metabolism in perfused skeletal muscle. Effect of dichloroacetate. Diabetes. 1978 Nov;27(11):1065–1074. doi: 10.2337/diab.27.11.1065. [DOI] [PubMed] [Google Scholar]
- Goodman M. N., Ruderman N. B. Insulin sensitivity of rat skeletal muscle: effects of starvation and aging. Am J Physiol. 1979 May;236(5):E519–E523. doi: 10.1152/ajpendo.1979.236.5.E519. [DOI] [PubMed] [Google Scholar]
- Hermansen L., Hultman E., Saltin B. Muscle glycogen during prolonged severe exercise. Acta Physiol Scand. 1967 Oct-Nov;71(2):129–139. doi: 10.1111/j.1748-1716.1967.tb03719.x. [DOI] [PubMed] [Google Scholar]
- Hickson R. C., Heusner W. W., Van Huss W. D., Taylor J. F., Carrow R. E. Effects of an anabolic steroid and sprint training on selected histochemical and morphological observations in rat skeletal muscle types. Eur J Appl Physiol Occup Physiol. 1976 Sep 23;35(4):251–259. doi: 10.1007/BF00423284. [DOI] [PubMed] [Google Scholar]
- Kahn C. R. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism. 1978 Dec;27(12 Suppl 2):1893–1902. doi: 10.1016/s0026-0495(78)80007-9. [DOI] [PubMed] [Google Scholar]
- Karlsson J., Diamant B., Saltin B. Muscle metabolites during submaximal and maximal exercise in man. Scand J Clin Lab Invest. 1970 Dec;26(4):385–394. doi: 10.3109/00365517009046250. [DOI] [PubMed] [Google Scholar]
- Kochan R. G., Lamb D. R., Lutz S. A., Perrill C. V., Reimann E. M., Schlender K. K. Glycogen synthase activation in human skeletal muscle: effects of diet and exercise. Am J Physiol. 1979 Jun;236(6):E660–E666. doi: 10.1152/ajpendo.1979.236.6.E660. [DOI] [PubMed] [Google Scholar]
- Kochan R. G., Lamb D. R., Reimann E. M., Schlender K. K. Modified assays to detect activation of glycogen synthase following exercise. Am J Physiol. 1981 Feb;240(2):E197–E202. doi: 10.1152/ajpendo.1981.240.2.E197. [DOI] [PubMed] [Google Scholar]
- Koivisto V. A., Soman V. R., Felig P. Effects of acute exercise on insulin binding to monocytes in obesity. Metabolism. 1980 Feb;29(2):168–172. doi: 10.1016/0026-0495(80)90142-0. [DOI] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Freychet P. Effect of fasting and streptozotocin diabetes on insulin binding and action in the isolated mouse soleus muscle. J Clin Invest. 1979 Nov;64(5):1505–1515. doi: 10.1172/JCI109609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehlum S., Felig P., Wahren J. Splanchnic glucose and muscle glycogen metabolism after glucose feeding during postexercise recovery. Am J Physiol. 1978 Sep;235(3):E255–E260. doi: 10.1152/ajpendo.1978.235.3.E255. [DOI] [PubMed] [Google Scholar]
- Maizels E. Z., Ruderman N. B., Goodman M. N., Lau D. Effect of acetoacetate on glucose metabolism in the soleus and extensor digitorum longus muscles of the rat. Biochem J. 1977 Mar 15;162(3):557–568. doi: 10.1042/bj1620557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen O., Beck-Nielsen H., Heding L. Increased insulin receptors after exercise in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1980 Apr 17;302(16):886–892. doi: 10.1056/NEJM198004173021603. [DOI] [PubMed] [Google Scholar]
- Pernow B., Saltin B. Availability of substrates and capacity for prolonged heavy exercise in man. J Appl Physiol. 1971 Sep;31(3):416–422. doi: 10.1152/jappl.1971.31.3.416. [DOI] [PubMed] [Google Scholar]
- Piras R., Staneloni R. In vivo regulation of rat muscle glycogen synthetase activity. Biochemistry. 1969 May;8(5):2153–2160. doi: 10.1021/bi00833a056. [DOI] [PubMed] [Google Scholar]
- Pruett E. D., Oseid S. Effect of exercise on glucose and insulin response to glucose infusion. Scand J Clin Lab Invest. 1970 Nov;26(3):277–285. doi: 10.3109/00365517009046234. [DOI] [PubMed] [Google Scholar]
- Rennie M. J., Holloszy J. O. Inhibition of glucose uptake and glycogenolysis by availability of oleate in well-oxygenated perfused skeletal muscle. Biochem J. 1977 Nov 15;168(2):161–170. doi: 10.1042/bj1680161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter E. A., Ruderman N. B., Schneider S. H. Diabetes and exercise. Am J Med. 1981 Jan;70(1):201–209. doi: 10.1016/0002-9343(81)90427-7. [DOI] [PubMed] [Google Scholar]
- Roach R. J., Larner J. Covalent phosphorylation in the regulation glycogen synthase activity. Mol Cell Biochem. 1977 May 3;15(3):179–200. doi: 10.1007/BF01734108. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Goodman M. N., Berger M., Hagg S. Effect of starvation on muscle glucose metabolism: studies with the isolated perfused rat hindquarter. Fed Proc. 1977 Feb;36(2):171–176. [PubMed] [Google Scholar]
- Ruderman N. B., Houghton C. R., Hems R. Evaluation of the isolated perfused rat hindquarter for the study of muscle metabolism. Biochem J. 1971 Sep;124(3):639–651. doi: 10.1042/bj1240639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Kemmer F. W., Goodman M. N., Berger M. Oxygen consumption in perfused skeletal muscle. Effect of perfusion with aged, fresh and aged-rejuvenated erythrocytes on oxygen consumption, tissue metabolites and inhibition of glucose utilization by acetoacetate. Biochem J. 1980 Jul 15;190(1):57–64. doi: 10.1042/bj1900057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Wahren J., Felig P., Hendler R., Ahlborg G. Glucose and amino acid metabolism during recovery after exercise. J Appl Physiol. 1973 Jun;34(6):838–845. doi: 10.1152/jappl.1973.34.6.838. [DOI] [PubMed] [Google Scholar]


