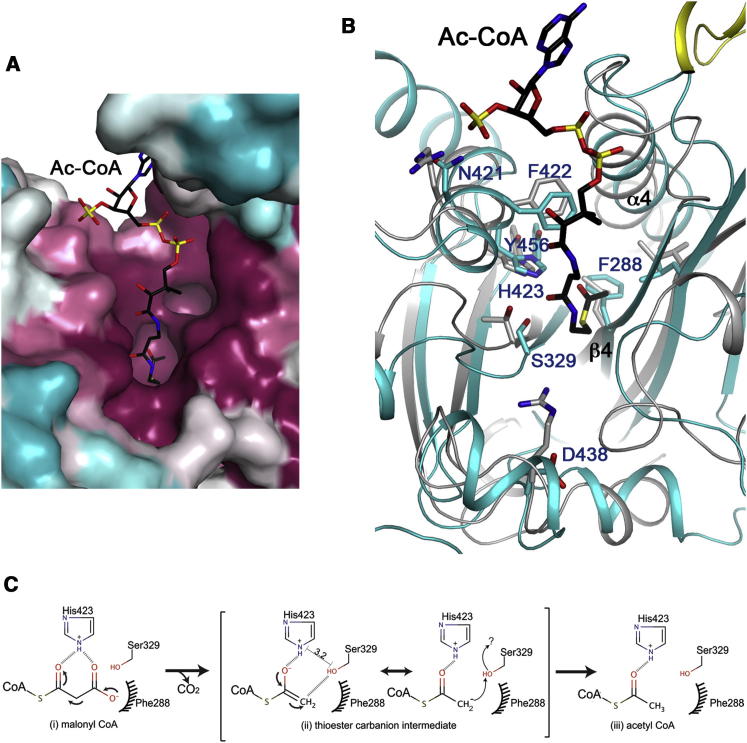
Figure 5.
The Active Site of MCD
(A) Molecular surface of HsMCD in the active site region, colored by sequence conservation (magenta, most conserved; cyan, least conserved). The bound position of acetyl-CoA in CurA (Gu et al., 2007) is shown as a stick model (in black).
(B) An overlay of HsMCD (in color) and CurA (in gray) in the active site region. Side chains in HsMCD are labeled. The catalytic residues His423 and Ser329 of HsMCD are equivalent to His389 and Thr335 of CurA. Please see Figure S3 for a stereo version of this panel.
(C) Proposed catalytic mechanism for MCD (HsMCD numbering). Interatomic distance between His423 imidazole nitrogen and Ser329 hydroxyl oxygen is denoted in black line. Question mark represents possible proton transfer to reprotonate Ser329, from His423, a water molecule, or other unidentified sources.
See also Figure S3.