Figure 3.
Plasma Cholesterol Level and Atherosclerotic Plaque in Simvastatin- and Acipimox-Treated ApoE−/− Mice; Cold-Induced Alterations of BAT Activation, BMI, Metabolism, Blood Lipid Profile, Atherosclerotic Plaque Growth, and Stability in ApoE−/−/UCP1−/− Mice
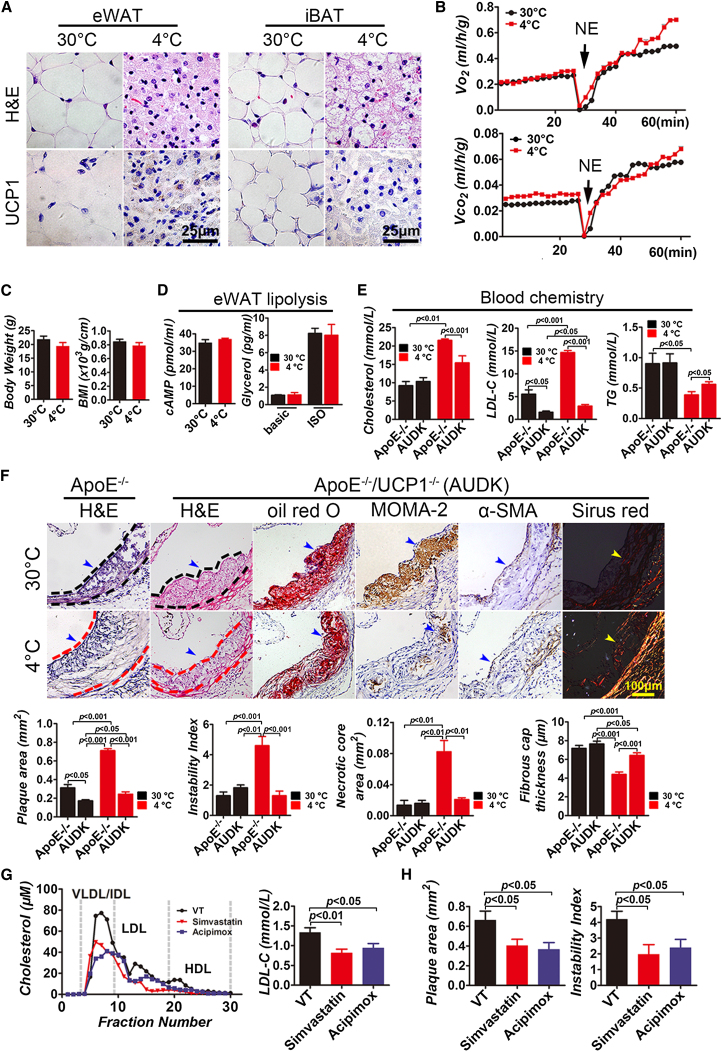
(A) Histology and lack of UCP1 expression in eWAT and iBAT exposed to 4°C and 30°C for 4 weeks.
(B) Metabolic rate of O2 consumption and CO2 production in response to NE (n = 5–6/group). No statistical significance between the two groups at any time points.
(C) Body weight and BMI of pair-fed ApoE−/−/UCP1−/− mice exposed to 4°C and 30°C (n = 6–7/group), showing no statistical significance between the two groups.
(D) Measurement of cAMP and glycerol levels released from eWAT of ApoE−/−/UCP1−/− mice exposed to 4°C and 30°C for 4 weeks (n = 4/group). No statistical significance between the two groups.
(E) Blood chemistry analysis of cholesterol, LDL cholesterol, and TG levels of ApoE−/−/UCP1−/− or ApoE−/− mice exposed to 4°C and 30°C (n = 6/group).
(F) Histological analysis of aorta roots of ApoE−/−/UCP1−/− mice exposed to 4°C and 30°C for 4 weeks by staining with H&E, oil red O, MOMA-2, α-SMA, or Sirius red. H&E staining of the aorta roots of ApoE−/− mice exposed to 4°C and 30°C for 4 weeks was used as a control. Dashed lines encircle some parts of atherosclerotic plaques, and arrowheads in different panels point to positive signals (n = 6–7/group). Quantification of plaque area, instability index, necrotic core areas, and fibrous cap thickness in ApoE−/−/UCP1−/− and ApoE−/− mice (n = 6–7/group).
(G) FPLC analysis of plasma cholesterol in vehicle-, simvastatin- and acipimox-treated apoE−/− mice (n = 5/group).
(H) Quantification of plaque areas and instability index of vehicle-, statin-, and acipimox-treated groups (n = 10/group). VT, vehicle-treated; NE, norepinephrine; AUDK, ApoE−/−/UCP1−/−.