Figure 1.
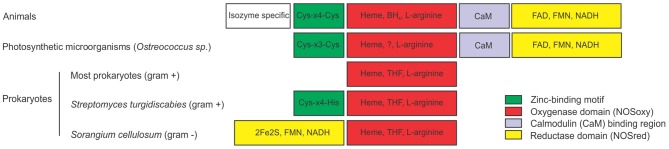
Structures of nitric oxide synthases (NOSs) from different sources. Comparison of animal NOS structure with NOSs from photosynthetic microorganisms and prokaryotes. Animal NOSs contain a zinc-binding region (Cys-X4-Cys), a NOS oxygenase domain (NOSoxy) which binds Heme, arginine and BH4, a calmodulin-binding region (CaM) and a NOS reductase domain (NOSred), which binds FMN, FAD, and NAD. The only described NOS of the photosynthetic organism is from the Ostreococcus genus. It has the NOSoxy, NOSred, a partially conserved CaM domain and a Zn-binding motif that partially differs from animals (Cys-X3-Cys). Most prokaryotes has only the NOSoxy domain, with the exception for the gram negative bacterium Sorangium cellullosum that has a novel NOSred domain in the N-terminal of the protein containing a 2Fe2S ferredoxin subdomain. Streptomyces turgidiscabies also has a partially conserved zinc binding motif (Cys-X4-His). Most prokaryotes produce tetrahydrofolate (THF) instead of the cofactor BH4. ? indicates that the co-factor that replaces BH4 in Ostreococcus is unknown.
