Abstract
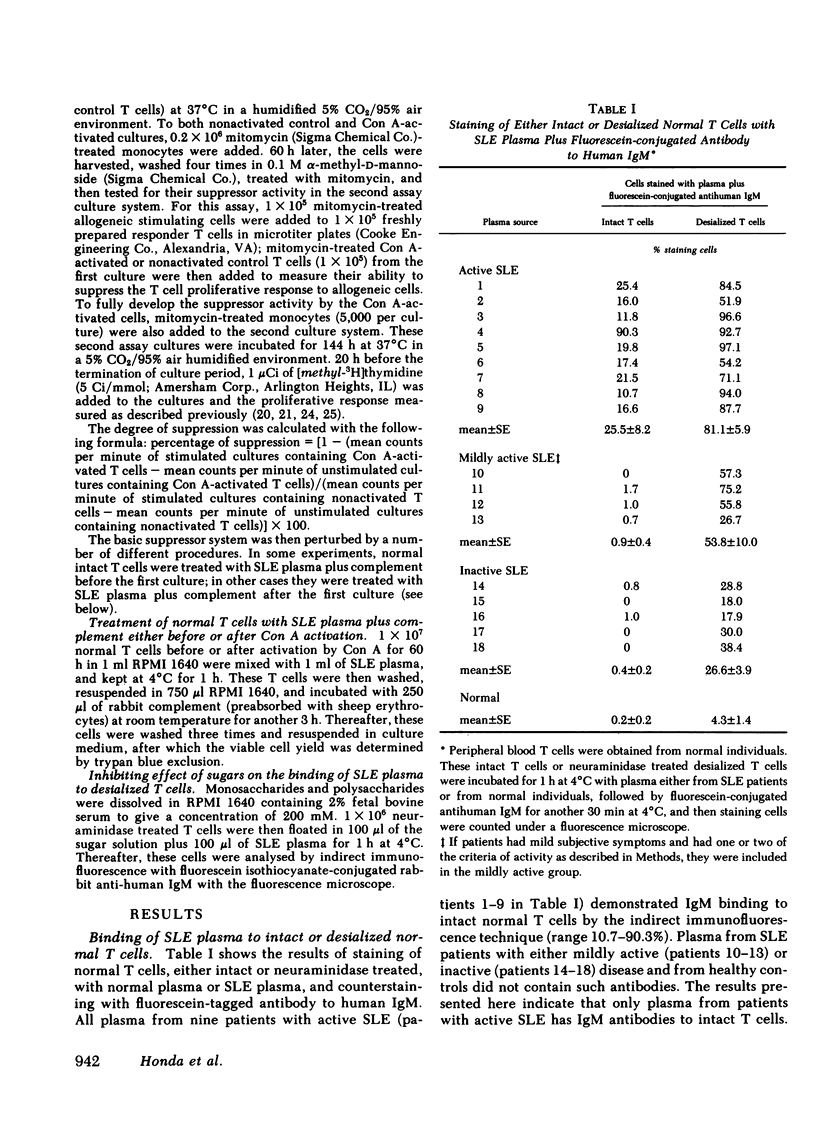
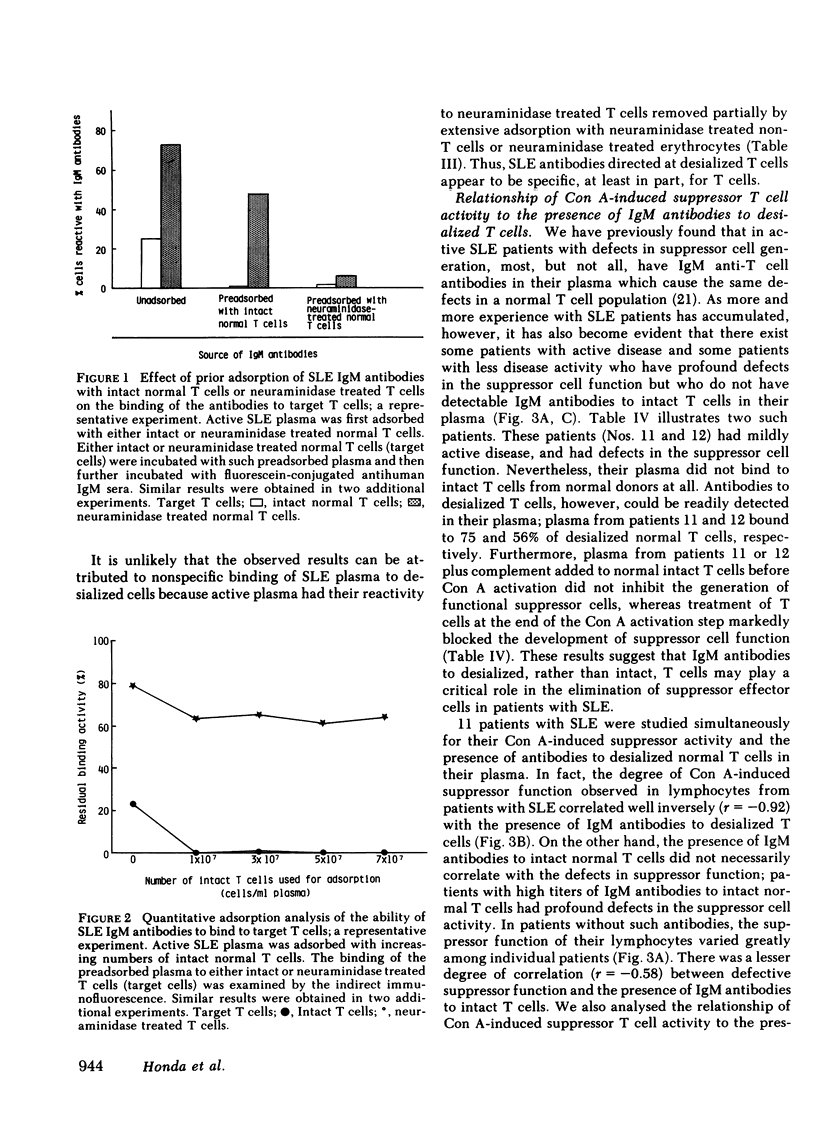
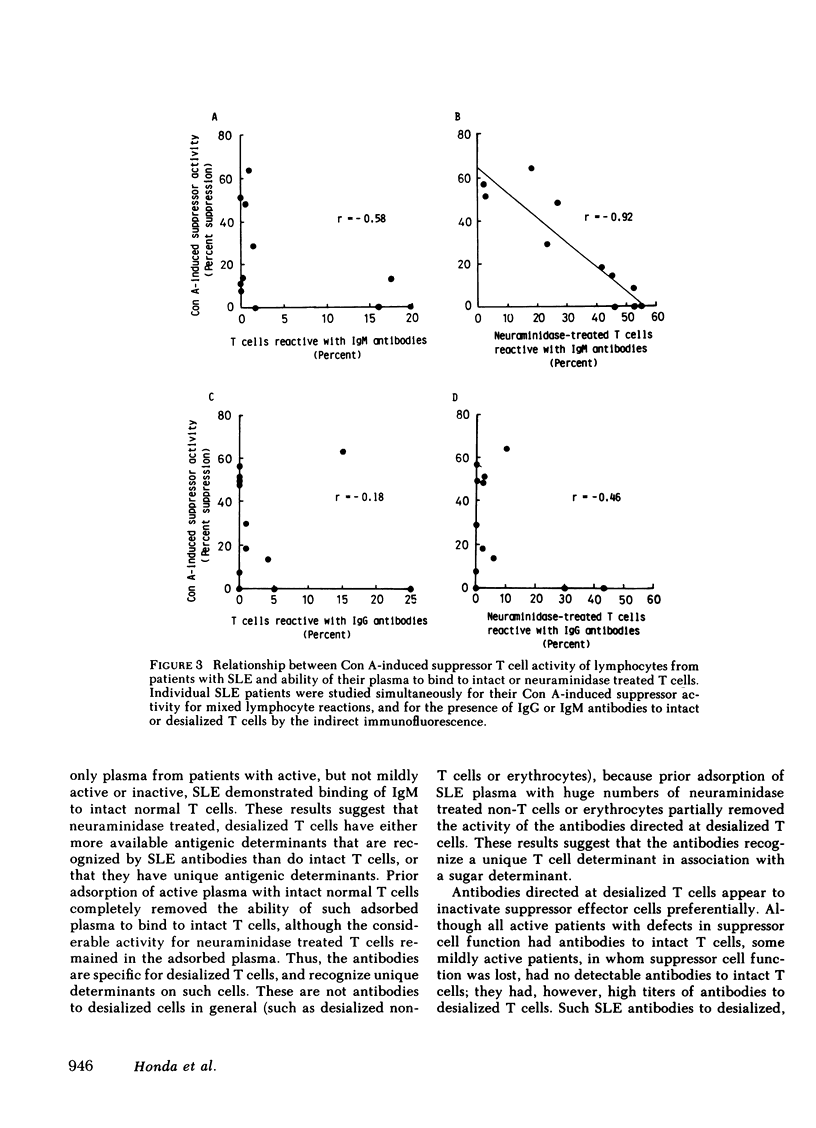
Patients with systemic lupus erythematosus (SLE) were found to have in their plasma antibodies specific for desialized T cells. Adsorption studies with intact or desialized T cells indicated that SLE anti-T cell antibodies consisted of two populations with different target cell specificities, one capable of recognizing unique determinants on desialized T cells and another able to bind to both intact and desialized T cells. Normal T cells did not remove the antibodies specific for desialized T cells. moreover, the antibodies to desialized T cells were not removed by adsorption with either desialized non-T cells or desialized erythrocytes. Thus, the antibodies to desialized T cells recognize a determinant that is unique to a T cell subset and also includes a sugar. Inhibition studies with various sugars indicated that lactose was the most potent inhibitor of antibody binding. The anti-desialized T cell antibody appears to recognize a T cell determinant which includes lactose, probably in the form of a beta-galactosyl residue, but which also includes additional T cell determinants. The antibodies to desialized T cells were found to bind preferentially to concanavalin A-induced autorosetting T cells, which had been already demonstrated to contain suppressor effector cells. Indeed, such antibodies were effective in eliminating suppressor effector function without interfering with T cells necessary for such activation (such as precursor or inducer cells). Finally, studies of patients with SLE yielded a highly significant correlation (r = 0.92) between impaired suppressor effector function of their cells and the presence of antibodies to desialized T cells in their plasma.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Erb P., Meier B., Kraus D., von Boehmer H., Feldmann M. Nature of T cell-macrophage interaction in helper cell induction in vitro. I. Evidence for genetic restriction of T cell-macrophage interactions prior to T cell priming. Eur J Immunol. 1978 Nov;8(11):786–792. doi: 10.1002/eji.1830081107. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Steinberg A. D., Haynes B. F., Whalen G. Immunoregulatory aberrations in systemic lupus erythematosus. J Immunol. 1978 Oct;121(4):1473–1479. [PubMed] [Google Scholar]
- Fournier C., Charreire J. Activation of a human T cell subpopulation bearing receptors for autologous erythrocytes by concanavalin A. J Immunol. 1978 Aug;121(2):771–776. [PubMed] [Google Scholar]
- Gershon R. K. T cell control of antibody production. Contemp Top Immunobiol. 1974;3:1–40. doi: 10.1007/978-1-4684-3045-5_1. [DOI] [PubMed] [Google Scholar]
- Glinski W., Gershwin M. E., Steinberg A. D. Fractionation of cells on a discontinuous Ficoll gradient. Study of subpopulations of human T cells using anti-T-cell antibodies from patients with systemic lupus erythematosus. J Clin Invest. 1976 Mar;57(3):604–614. doi: 10.1172/JCI108316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz S., Borcherding W., Moorthy A. V., Chesney R., Schulte-Wisserman H., Hong R. Induction of suppressor T cells in systemic lupus erythematosus by thymosin and cultured thymic epithelium. Science. 1977 Sep 2;197(4307):999–1001. doi: 10.1126/science.302032. [DOI] [PubMed] [Google Scholar]
- Imai Y., Nakano T., Sawada J. I., Osawa T. Specificity of natural thymocytotoxic autoantibody developed in New Zealand black mice. J Immunol. 1980 Apr;124(4):1556–1561. [PubMed] [Google Scholar]
- Kumagai S., Steinberg A. D., Green I. Antibodies to T cells in patients with systemic lupus erythematosus can induce antibody-dependent cell-mediated cytotoxicity against human T cells. J Clin Invest. 1981 Mar;67(3):605–614. doi: 10.1172/JCI110074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lies R. B., Messner R. P., Williams R. C., Jr Relative T-cell specificity of lymphocytotoxins from patients with systemic lupus erythematosus. Arthritis Rheum. 1973 May-Jun;16(3):369–375. doi: 10.1002/art.1780160312. [DOI] [PubMed] [Google Scholar]
- Nakano T., Imai Y., Naiki M., Osawa T. Characterization of mouse helper and suppressor T cell subsets separated by lectins. J Immunol. 1980 Nov;125(5):1928–1932. [PubMed] [Google Scholar]
- Nakano T., Oguchi Y., Imai Y., Osawa T. Induction and separation of mouse helper T cells by lectins. Immunology. 1980 Jun;40(2):217–222. [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Reisner Y., Biniaminov M., Rosenthal E., Sharon N., Ramot B. Interaction of peanut agglutinin with normal human lymphocytes and with leukemic cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):447–451. doi: 10.1073/pnas.76.1.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner Y., Linker-Israeli M., Sharon N. Separation of mouse thymocytes into two subpopulations by the use of peanut agglutinin. Cell Immunol. 1976 Jul;25(1):129–134. doi: 10.1016/0008-8749(76)90103-9. [DOI] [PubMed] [Google Scholar]
- Sagawa A., Abdou N. I. Suppressor-cell antibody in systemic lupus erythematosus. Possible mechanism for suppressor-cell dysfunction. J Clin Invest. 1979 Mar;63(3):536–539. doi: 10.1172/JCI109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagawa A., Abdou N. I. Suppressor-cell dysfunction in systemic lupus erythematosus. Cells involved and in vitro correction. J Clin Invest. 1978 Oct;62(4):789–796. doi: 10.1172/JCI109190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Honda M., Taniguchi Y., Kotani H. Separation of concanavalin A-induced human suppressor and helper T cells by the autologous erythrocyte rosette technique. J Clin Invest. 1981 Aug;68(2):447–453. doi: 10.1172/JCI110274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Arnett F. C., Reinertsen J. L., Green I. Studies of immune functions of patients with systemic lupus erythematosus. Arthritis Rheum. 1979 Jul;22(7):770–776. doi: 10.1002/art.1780220713. [DOI] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Studies of immune functions of patients with systemic lupus erythematosus. I. Dysfunction of suppressor T-cell activity related to impaired generation of, rather than response to, suppressor cells. Arthritis Rheum. 1978 Jul-Aug;21(6):657–664. doi: 10.1002/art.1780210608. [DOI] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Studies of immune functions of patients with systemic lupus erythematosus. V. T cell suppressor function and autologous mixed lymphocyte reaction during active and inactive phases of disease. Arthritis Rheum. 1980 Feb;23(2):225–231. doi: 10.1002/art.1780230214. [DOI] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Reeves J. P., Green I. Studies of immune functions of patients with systemic lupus erythematosus. Complement-dependent immunoglobulin M anti-thymus-derived cell antibodies preferentially inactivate suppressor cells. J Clin Invest. 1979 May;63(5):954–965. doi: 10.1172/JCI109396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Reeves J. P., Green I. Studies of immune functions of patients with systemic lupus erythematosus. T-cell subsets and antibodies to T-cell subsets. J Clin Invest. 1979 Nov;64(5):1260–1269. doi: 10.1172/JCI109581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stastny P., Ziff M. Direct lysis of lymphocytes by complement in patients with systemic lupus erythematosus. Arthritis Rheum. 1971 Nov-Dec;14(6):733–736. doi: 10.1002/art.1780140608. [DOI] [PubMed] [Google Scholar]
- Steinberg A. D., Klassen L. W., Budman D. R., Williams G. W. Immunofluorescence studies of anti-T cell antibodies and T cells in systemic lupus erythematosus. Selective loss of brightly staining T cells in active disease. Arthritis Rheum. 1979 Feb;22(2):114–122. doi: 10.1002/art.1780220203. [DOI] [PubMed] [Google Scholar]
- Twomey J. J., Laughter A. H., Steinberg A. D. A serum inhibitor of immune regulation in patients with systemic lupus erythematosus. J Clin Invest. 1978 Sep;62(3):713–715. doi: 10.1172/JCI109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernet P., Kunkel H. G. Antibodies to a specific surface antigen of T cells in human sera inhibiting mixed leukocyte culture reactions. J Exp Med. 1973 Oct 1;138(4):1021–1026. doi: 10.1084/jem.138.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfield J. B., Winchester R. J., Wernet P., Fu S. M., Kunkel H. G. Nature of cold-reactive antibodies to lymphocyte surface determinants in systemic lupus erythematosus. Arthritis Rheum. 1975 Jan-Feb;18(1):1–8. doi: 10.1002/art.1780180101. [DOI] [PubMed] [Google Scholar]


